Analysis of the functional consequences of targeted exon deletion in COL7A1 reveals prospects for dystrophic epidermolysis bullosa therapy
- PMID: 27157667
- PMCID: PMC5088769
- DOI: 10.1038/mt.2016.92
Analysis of the functional consequences of targeted exon deletion in COL7A1 reveals prospects for dystrophic epidermolysis bullosa therapy
Abstract
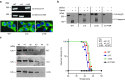
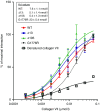
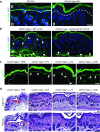
Genetically evoked deficiency of collagen VII causes dystrophic epidermolysis bullosa (DEB)-a debilitating disease characterized by chronic skin fragility and progressive fibrosis. Removal of exons carrying frame-disrupting mutations can reinstate protein expression in genetic diseases. The therapeutic potential of this approach is critically dependent on gene, protein, and disease intrinsic factors. Naturally occurring exon skipping in COL7A1, translating collagen VII, suggests that skipping of exons containing disease-causing mutations may be feasible for the treatment of DEB. However, despite a primarily in-frame arrangement of exons in the COL7A1 gene, no general conclusion of the aptitude of exon skipping for DEB can be drawn, since regulation of collagen VII functionality is complex involving folding, intra- and intermolecular interactions. To directly address this, we deleted two conceptually important exons located at both ends of COL7A1, exon 13, containing recurrent mutations, and exon 105, predicted to impact folding. The resulting recombinantly expressed proteins showed conserved functionality in biochemical and in vitro assays. Injected into DEB mice, the proteins promoted skin stability. By demonstrating functionality of internally deleted collagen VII variants, our study provides support of targeted exon deletion or skipping as a potential therapy to treat a large number of individuals with DEB.
Figures



References
-
- Bruckner-Tuderman, L (2010). Dystrophic epidermolysis bullosa: pathogenesis and clinical features. Dermatol Clin 28: 107–114. - PubMed
-
- Mittapalli, VR, Madl, J, Löffek, S, Kiritsi, D, Kern, JS, Römer, W et al. (2016). Injury-driven stiffening of the dermis expedites skin carcinoma progression. Cancer Res 76: 940–951. - PubMed
-
- Goto, M, Sawamura, D, Ito, K, Abe, M, Nishie, W, Sakai, K et al. (2006). Fibroblasts show more potential as target cells than keratinocytes in COL7A1 gene therapy of dystrophic epidermolysis bullosa. J Invest Dermatol 126: 766–772. - PubMed
-
- Goto, M, Sawamura, D, Nishie, W, Sakai, K, McMillan, JR, Akiyama, M et al. (2006). Targeted skipping of a single exon harboring a premature termination codon mutation: implications and potential for gene correction therapy for selective dystrophic epidermolysis bullosa patients. J Invest Dermatol 126: 2614–2620. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

