Microfluidic platform combining droplets and magnetic tweezers: application to HER2 expression in cancer diagnosis
- PMID: 27157697
- PMCID: PMC4860594
- DOI: 10.1038/srep25540
Microfluidic platform combining droplets and magnetic tweezers: application to HER2 expression in cancer diagnosis
Abstract
The development of precision medicine, together with the multiplication of targeted therapies and associated molecular biomarkers, call for major progress in genetic analysis methods, allowing increased multiplexing and the implementation of more complex decision trees, without cost increase or loss of robustness. We present a platform combining droplet microfluidics and magnetic tweezers, performing RNA purification, reverse transcription and amplification in a fully automated and programmable way, in droplets of 250nL directly sampled from a microtiter-plate. This platform decreases sample consumption about 100 fold as compared to current robotized platforms and it reduces human manipulations and contamination risk. The platform's performance was first evaluated on cell lines, showing robust operation on RNA quantities corresponding to less than one cell, and then clinically validated with a cohort of 21 breast cancer samples, for the determination of their HER2 expression status, in a blind comparison with an established routine clinical analysis.
Conflict of interest statement
The authors declare no competing financial interests.
Figures


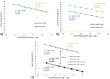

 obtained by the Saint-Louis Hospital platform versus the
obtained by the Saint-Louis Hospital platform versus the  obtained by the droplet microfluidic platform. Samples disclosed a posteriori as HER2- samples are plotted in red, and HER2+ ones in black. The dashed lines help to identify the excluded zone between the two populations: blue for the droplet platform (10.48–39.67), green for the hospital (1.5–11.71).
obtained by the droplet microfluidic platform. Samples disclosed a posteriori as HER2- samples are plotted in red, and HER2+ ones in black. The dashed lines help to identify the excluded zone between the two populations: blue for the droplet platform (10.48–39.67), green for the hospital (1.5–11.71).References
-
- Garraway L. A., Verweij J. & Ballman K. V. Precision oncology: an overview. J. Clin. Oncol. 31, 1803–5 (2013). - PubMed
-
- Stahel R. et al. Optimising translational oncology in clinical practice: strategies to accelerate progress in drug development. Cancer Treat. Rev. 41, 129–35 (2015). - PubMed
-
- André F. et al. Comparative genomic hybridisation array and DNA sequencing to direct treatment of metastatic breast cancer: a multicentre, prospective trial (SAFIR01/UNICANCER). Lancet. Oncol. 15, 267–74 (2014). - PubMed
-
- Swanton C. SAFIR01: steps towards precision treatment in breast cancer. Lancet. Oncol. 15, 242–3 (2014). - PubMed
-
- Wolff A. C. et al. Recommendations for human epidermal growth factor receptor 2 testing in breast cancer: American Society of Clinical Oncology/College of American Pathologists clinical practice guideline update. J. Clin. Oncol. 31, 3997–4013 (2013). - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

