Acute administration of ivacaftor to people with cystic fibrosis and a G551D-CFTR mutation reveals smooth muscle abnormalities
- PMID: 27158673
- PMCID: PMC4855508
- DOI: 10.1172/jci.insight.86183
Acute administration of ivacaftor to people with cystic fibrosis and a G551D-CFTR mutation reveals smooth muscle abnormalities
Abstract
Background: Airflow obstruction is common in cystic fibrosis (CF), yet the underlying pathogenesis remains incompletely understood. People with CF often exhibit airway hyperresponsiveness, CF transmembrane conductance regulator (CFTR) is present in airway smooth muscle (ASM), and ASM from newborn CF pigs has increased contractile tone, suggesting that loss of CFTR causes a primary defect in ASM function. We hypothesized that restoring CFTR activity would decrease smooth muscle tone in people with CF.
Methods: To increase or potentiate CFTR function, we administered ivacaftor to 12 adults with CF with the G551D-CFTR mutation; ivacaftor stimulates G551D-CFTR function. We studied people before and immediately after initiation of ivacaftor (48 hours) to minimize secondary consequences of CFTR restoration. We tested smooth muscle function by investigating spirometry, airway distensibility, and vascular tone.
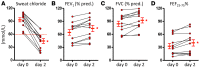
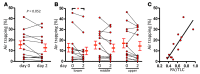
Results: Ivacaftor rapidly restored CFTR function, indicated by reduced sweat chloride concentration. Airflow obstruction and air trapping also improved. Airway distensibility increased in airways less than 4.5 mm but not in larger-sized airways. To assess smooth muscle function in a tissue outside the lung, we measured vascular pulse wave velocity (PWV) and augmentation index, which both decreased following CFTR potentiation. Finally, change in distensibility of <4.5-mm airways correlated with changes in PWV.
Conclusions: Acute CFTR potentiation provided a unique opportunity to investigate CFTR-dependent mechanisms of CF pathogenesis. The rapid effects of ivacaftor on airway distensibility and vascular tone suggest that CFTR dysfunction may directly cause increased smooth muscle tone in people with CF and that ivacaftor may relax smooth muscle.
Funding: This work was funded in part from an unrestricted grant from the Vertex Investigator-Initiated Studies Program.
Figures




References
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

