Clostridium difficile infection
- PMID: 27158839
- PMCID: PMC5453186
- DOI: 10.1038/nrdp.2016.20
Clostridium difficile infection
Abstract
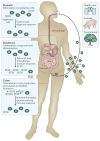
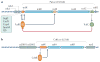
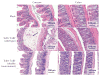
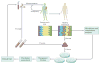
Infection of the colon with the Gram-positive bacterium Clostridium difficile is potentially life threatening, especially in elderly people and in patients who have dysbiosis of the gut microbiota following antimicrobial drug exposure. C. difficile is the leading cause of health-care-associated infective diarrhoea. The life cycle of C. difficile is influenced by antimicrobial agents, the host immune system, and the host microbiota and its associated metabolites. The primary mediators of inflammation in C. difficile infection (CDI) are large clostridial toxins, toxin A (TcdA) and toxin B (TcdB), and, in some bacterial strains, the binary toxin CDT. The toxins trigger a complex cascade of host cellular responses to cause diarrhoea, inflammation and tissue necrosis - the major symptoms of CDI. The factors responsible for the epidemic of some C. difficile strains are poorly understood. Recurrent infections are common and can be debilitating. Toxin detection for diagnosis is important for accurate epidemiological study, and for optimal management and prevention strategies. Infections are commonly treated with specific antimicrobial agents, but faecal microbiota transplants have shown promise for recurrent infections. Future biotherapies for C. difficile infections are likely to involve defined combinations of key gut microbiota.
Conflict of interest statement
W.K.S. has performed research for Cubist. D.L. has performed research for Immuron and Adenium Biotech. D.B.L. has performed research for MedImmune and Merck. M.H.W. has received consulting fees from Abbott, Actelion Pharmaceuticals, Astellas, AstraZeneca, Bayer, Cerexa, Cubist, Durata, The European Tissue Symposium, The Medicines Company, MedImmune, Merck, Motif Biosciences, Nabriva, Optimer, Paratek, Pfizer, Roche, Sanofi Pasteur, Seres Therapeutics, Summit Pharmaceuticals, and Synthetic Biologics. M.H.W. has also received lecture fees from Abbott, Alere, Astellas, AstraZeneca, Pfizer and Hoffmann La Roche, and received grant support from Abbott, Actelion, Astellas, bioMérieux, Cubist, Da Volterra, The European Tissue Symposium, Merck and Summit Pharmaceuticals. E.J.K. has performed research for Cubist, Novartis and Qiagen, and has participated in advisory forums of Astellas, Optimer, Actelion, Pfizer, Sanofi Pasteur and Seres Therapeutics. These companies had no role in the writing of this Primer.
Figures






References
-
- Hall IC, O’Toole E. Intestinal flora in new-born infants: with a description of a new pathogenic anaerobe, Bacillus difficilis. Am J Child Dis. 1935;49:390–402.
-
- Kuehne SA, et al. The role of toxin A and toxin B in Clostridium difficile infection. Nature. 2010;467:711–713. - PubMed
-
- Hafiz S, Oakley CL. Clostridium difficile: isolation and characteristics. J Med Microbiol. 1976;9:129–136. - PubMed
-
- Bartlett JG. Clostridium difficile: history of its role as an enteric pathogen and the current state of knowledge about the organism. Clin Infect Dis. 1994;18:S265–S272. An exceptional overview of the early experiments demonstrating the involvement of C. difficile in (antibiotic-associated) colitis. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

