Imbalance of Amniotic Fluid Activin-A and Follistatin in Intraamniotic Infection, Inflammation, and Preterm Birth
- PMID: 27159193
- PMCID: PMC6287504
- DOI: 10.1210/jc.2015-4147
Imbalance of Amniotic Fluid Activin-A and Follistatin in Intraamniotic Infection, Inflammation, and Preterm Birth
Abstract
Context: Microbial invasion of the amniotic fluid (AF) cavity stimulates an inflammatory response that involves activin-A, a pleiotropic mediator member of the TGFβ superfamily involved in connective tissue remodeling. The role of AF follistatin, a natural inhibitor of activin-A, in inflammation-induced preterm birth (PTB), has yet to be determined.
Objective: The objective of the study was to investigate the relationships between AF activin-A and follistatin in physiological gestation and in pregnancies complicated by PTB and to evaluate a possible role played by the activin-A-follistatin balance in processes leading to PTB and preterm premature rupture of membranes (PPROM).
Study design: The AF levels of total activin-A and follistatin were immunoassayed in 168 women with a normal pregnancy outcome or PTB with and without intraamniotic inflammation or PPROM. The impact of the activin-A-follistatin imbalance on PTB terminal effector pathways (prostaglandins [prostaglandin E2, prostaglandin F2α] and matrix metalloproteinases [MMP-1, MMP-2, MMP-3, and MMP-9]) was investigated in an amniochorion explant system challenged with lipopolysaccharide (LPS) to mimic inflammation.
Results: AF follistatin and the activin-A to follistatin ratio varied with gestational age, both decreasing toward term (P < .001). Activin-A was up-regulated in AF infection (>2-fold elevation in activin-A to follistatin ratio) correlating directly with severity of inflammation (both P < .001). Activin-A increased prostaglandins, MMP-1, and MMP-9 released by amniochorion (P < .05) to LPS-equivalent levels. Follistatin effectively blunted the prostaglandin response to activin-A and LPS and that of MMPs after activin-A but not after LPS challenge.
Conclusion: Activin-A and follistatin are part of the complex inflammatory response of the gestational sac to infection and modulate effector pathways leading to PTB. The activin-A to follistatin ratio may play a role in determining the clinical phenotype of PTB as preterm labor or PPROM.
Figures



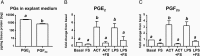

References
-
- de Kretser DM, O'Hehir RE, Hardy CL, Hedger MP. The roles of activin A and its binding protein, follistatin, in inflammation and tissue repair. Mol Cell Endocrinol. 2012;359:101–106. - PubMed
-
- de Winter JP, ten Dijke P, de Vries CJ, et al. . Follistatins neutralize activin bioactivity by inhibition of activin binding to its type II receptors. Mol Cell Endocrinol. 1996;116:105–114. - PubMed
-
- Schneyer AL, Wang Q, Sidis Y, Sluss PM. Differential distribution of follistatin isoforms: application of a new FS315-specific immunoassay. J Clin Endocrinol Metab. 2004;89:5067–5075. - PubMed
-
- Hedger MP, Winnall WR, Phillips DJ, de Kretser DM. The regulation and functions of activin and follistatin in inflammation and immunity. Vitam Horm. 2011;85:255–297. - PubMed
Publication types
MeSH terms
Substances
Supplementary concepts
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

