Age-Dependent Susceptibility to Enteropathogenic Escherichia coli (EPEC) Infection in Mice
- PMID: 27159323
- PMCID: PMC4861285
- DOI: 10.1371/journal.ppat.1005616
Age-Dependent Susceptibility to Enteropathogenic Escherichia coli (EPEC) Infection in Mice
Abstract
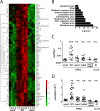
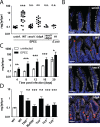
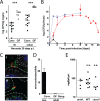
Enteropathogenic Escherichia coli (EPEC) represents a major causative agent of infant diarrhea associated with significant morbidity and mortality in developing countries. Although studied extensively in vitro, the investigation of the host-pathogen interaction in vivo has been hampered by the lack of a suitable small animal model. Using RT-PCR and global transcriptome analysis, high throughput 16S rDNA sequencing as well as immunofluorescence and electron microscopy, we characterize the EPEC-host interaction following oral challenge of newborn mice. Spontaneous colonization of the small intestine and colon of neonate mice that lasted until weaning was observed. Intimate attachment to the epithelial plasma membrane and microcolony formation were visualized only in the presence of a functional bundle forming pili (BFP) and type III secretion system (T3SS). Similarly, a T3SS-dependent EPEC-induced innate immune response, mediated via MyD88, TLR5 and TLR9 led to the induction of a distinct set of genes in infected intestinal epithelial cells. Infection-induced alterations of the microbiota composition remained restricted to the postnatal period. Although EPEC colonized the adult intestine in the absence of a competing microbiota, no microcolonies were observed at the small intestinal epithelium. Here, we introduce the first suitable mouse infection model and describe an age-dependent, virulence factor-dependent attachment of EPEC to enterocytes in vivo.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures



References
-
- Kotloff KL, Nataro JP, Blackwelder WC, Nasrin D, Farag TH, et al. (2013) Burden and aetiology of diarrhoeal disease in infants and young children in developing countries (the Global Enteric Multicenter Study, GEMS): a prospective, case-control study. Lancet 382: 209–222. 10.1016/S0140-6736(13)60844-2 - DOI - PubMed
-
- Cleary J, Lai LC, Shaw RK, Straatman-Iwanowska A, Donnenberg MS, et al. (2004) Enteropathogenic Escherichia coli (EPEC) adhesion to intestinal epithelial cells: role of bundle-forming pili (BFP), EspA filaments and intimin. Microbiology 150: 527–538. - PubMed
-
- Giron JA, Ho AS, Schoolnik GK (1991) An inducible bundle-forming pilus of enteropathogenic Escherichia coli. Science 254: 710–713. - PubMed
-
- Kenny B, DeVinney R, Stein M, Reinscheid DJ, Frey EA, et al. (1997) Enteropathogenic E. coli (EPEC) transfers its receptor for intimate adherence into mammalian cells. Cell 91: 511–520. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

