Immune activation and response to pembrolizumab in POLE-mutant endometrial cancer
- PMID: 27159395
- PMCID: PMC4887167
- DOI: 10.1172/JCI84940
Immune activation and response to pembrolizumab in POLE-mutant endometrial cancer
Abstract
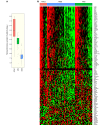
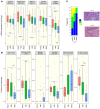
Antibodies that target the immune checkpoint receptor programmed cell death protein 1 (PD-1) have resulted in prolonged and beneficial responses toward a variety of human cancers. However, anti-PD-1 therapy in some patients provides no benefit and/or results in adverse side effects. The factors that determine whether patients will be drug sensitive or resistant are not fully understood; therefore, genomic assessment of exceptional responders can provide important insight into patient response. Here, we identified a patient with endometrial cancer who had an exceptional response to the anti-PD-1 antibody pembrolizumab. Clinical grade targeted genomic profiling of a pretreatment tumor sample from this individual identified a mutation in DNA polymerase epsilon (POLE) that associated with an ultramutator phenotype. Analysis of The Cancer Genome Atlas (TCGA) revealed that the presence of POLE mutation associates with high mutational burden and elevated expression of several immune checkpoint genes. Together, these data suggest that cancers harboring POLE mutations are good candidates for immune checkpoint inhibitor therapy.
Figures

References
-
- Rizvi NA, et al. Activity and safety of nivolumab, an anti-PD-1 immune checkpoint inhibitor, for patients with advanced, refractory squamous non-small-cell lung cancer (CheckMate 063): a phase 2, single-arm trial. Lancet Oncol. 2015;16(3):257–265. doi: 10.1016/S1470-2045(15)70054-9. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

