Association of MTOR Mutations With Developmental Brain Disorders, Including Megalencephaly, Focal Cortical Dysplasia, and Pigmentary Mosaicism
- PMID: 27159400
- PMCID: PMC4979321
- DOI: 10.1001/jamaneurol.2016.0363
Association of MTOR Mutations With Developmental Brain Disorders, Including Megalencephaly, Focal Cortical Dysplasia, and Pigmentary Mosaicism
Abstract
Importance: Focal cortical dysplasia (FCD), hemimegalencephaly, and megalencephaly constitute a spectrum of malformations of cortical development with shared neuropathologic features. These disorders are associated with significant childhood morbidity and mortality.
Objective: To identify the underlying molecular cause of FCD, hemimegalencephaly, and diffuse megalencephaly.
Design, setting, and participants: Patients with FCD, hemimegalencephaly, or megalencephaly (mean age, 11.7 years; range, 2-32 years) were recruited from Pediatric Hospital A. Meyer, the University of Hong Kong, and Seattle Children's Research Institute from June 2012 to June 2014. Whole-exome sequencing (WES) was performed on 8 children with FCD or hemimegalencephaly using standard-depth (50-60X) sequencing in peripheral samples (blood, saliva, or skin) from the affected child and their parents and deep (150-180X) sequencing in affected brain tissue. Targeted sequencing and WES were used to screen 93 children with molecularly unexplained diffuse or focal brain overgrowth. Histopathologic and functional assays of phosphatidylinositol 3-kinase-AKT (serine/threonine kinase)-mammalian target of rapamycin (mTOR) pathway activity in resected brain tissue and cultured neurons were performed to validate mutations.
Main outcomes and measures: Whole-exome sequencing and targeted sequencing identified variants associated with this spectrum of developmental brain disorders.
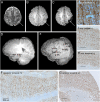
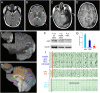
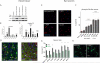
Results: Low-level mosaic mutations of MTOR were identified in brain tissue in 4 children with FCD type 2a with alternative allele fractions ranging from 0.012 to 0.086. Intermediate-level mosaic mutation of MTOR (p.Thr1977Ile) was also identified in 3 unrelated children with diffuse megalencephaly and pigmentary mosaicism in skin. Finally, a constitutional de novo mutation of MTOR (p.Glu1799Lys) was identified in 3 unrelated children with diffuse megalencephaly and intellectual disability. Molecular and functional analysis in 2 children with FCD2a from whom multiple affected brain tissue samples were available revealed a mutation gradient with an epicenter in the most epileptogenic area. When expressed in cultured neurons, all MTOR mutations identified here drive constitutive activation of mTOR complex 1 and enlarged neuronal size.
Conclusions and relevance: In this study, mutations of MTOR were associated with a spectrum of brain overgrowth phenotypes extending from FCD type 2a to diffuse megalencephaly, distinguished by different mutations and levels of mosaicism. These mutations may be sufficient to cause cellular hypertrophy in cultured neurons and may provide a demonstration of the pattern of mosaicism in brain and substantiate the link between mosaic mutations of MTOR and pigmentary mosaicism in skin.
Figures

Comment in
-
Genetic Gradients in Epileptic Brain Malformations.JAMA Neurol. 2016 Jul 1;73(7):787. doi: 10.1001/jamaneurol.2016.1039. JAMA Neurol. 2016. PMID: 27158974 No abstract available.
References
-
- Harvey AS, Cross JH, Shinnar S, Mathern BW. Defining the spectrum of international practice in pediatric epilepsy surgery patients. Epilepsia. 2008;49:146–55. - PubMed
-
- Ljungberg MC, Bhattacharjee MB, Lu Y, et al. Activation of mammalian target of rapamycin in cytomegalic neurons of human cortical dysplasia. Ann. Neurol. 2006;60(4):420–429. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

