Ensemble cryo-EM uncovers inchworm-like translocation of a viral IRES through the ribosome
- PMID: 27159452
- PMCID: PMC4896748
- DOI: 10.7554/eLife.14874
Ensemble cryo-EM uncovers inchworm-like translocation of a viral IRES through the ribosome
Abstract
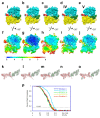
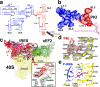
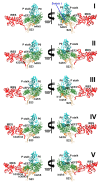
Internal ribosome entry sites (IRESs) mediate cap-independent translation of viral mRNAs. Using electron cryo-microscopy of a single specimen, we present five ribosome structures formed with the Taura syndrome virus IRES and translocase eEF2•GTP bound with sordarin. The structures suggest a trajectory of IRES translocation, required for translation initiation, and provide an unprecedented view of eEF2 dynamics. The IRES rearranges from extended to bent to extended conformations. This inchworm-like movement is coupled with ribosomal inter-subunit rotation and 40S head swivel. eEF2, attached to the 60S subunit, slides along the rotating 40S subunit to enter the A site. Its diphthamide-bearing tip at domain IV separates the tRNA-mRNA-like pseudoknot I (PKI) of the IRES from the decoding center. This unlocks 40S domains, facilitating head swivel and biasing IRES translocation via hitherto-elusive intermediates with PKI captured between the A and P sites. The structures suggest missing links in our understanding of tRNA translocation.
Keywords: IRES; S. cerevisiae; Taura syndrome virus; biochemistry; biophysics; elongation factor eEF2; internal ribosome entry site; ribosome; structural biology; translocation.
Conflict of interest statement
NG: Reviewing editor,
The other authors declare that no competing interests exist.
Figures




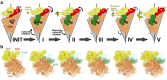
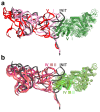




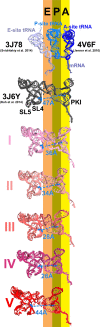




References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

