Mold-casted non-degradable, islet macro-encapsulating hydrogel devices for restoration of normoglycemia in diabetic mice
- PMID: 27159557
- PMCID: PMC11287382
- DOI: 10.1002/bit.26005
Mold-casted non-degradable, islet macro-encapsulating hydrogel devices for restoration of normoglycemia in diabetic mice
Abstract
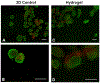
Islet transplantation is a potential cure for diabetic patients, however this procedure is not widely adopted due to the high rate of graft failure. Islet encapsulation within hydrogels is employed to provide a three-dimensional microenvironment conducive to survival of transplanted islets to extend graft function. Herein, we present a novel macroencapsulation device, composed of PEG hydrogel, that combines encapsulation with lithography techniques to generate polydimethylsiloxane (PDMS) molds. PEG solutions are mixed with islets, which are then cast into PDMS molds for subsequent crosslinking. The molds can also be employed to provide complex architectures, such as microchannels that may allow vascular ingrowth through pre-defined regions of the hydrogel. PDMS molds allowed for the formation of stable gels with encapsulation of islets, and in complex architectures. Hydrogel devices with a thickness of 600 μm containing 500 islets promoted normoglycemia within 12 days following transplantation into the epididymal fat pad, which was sustained over the two-month period of study until removal of the device. The inclusion of microchannels, which had a similar minimum distance between islets and the hydrogel surface, similarly promoted normoglycemia. A glucose challenge test indicated hydrogel devices achieved normoglycemia 90 min post-dextrose injections, similar to control mice with native pancreata. Histochemical staining revealed that transplanted islets, identified as insulin positive, were viable and isolated from host tissue at 8 weeks post-transplantation, yet devices with microchannels had tissue and vascular ingrowth within the channels. Taken together, these results demonstrate a system for creating non-degradable hydrogels with complex geometries for encapsulating islets capable of restoring normoglycemia, which may expand islet transplantation as a treatment option for diabetic patients. Biotechnol. Bioeng. 2016;113: 2485-2495. © 2016 Wiley Periodicals, Inc.
Keywords: encapsulation; hydrogel; macroencapsulation device; microchannels; polydimethylsiloxane (PDMS); polyethylene glycol (PEG).
© 2016 Wiley Periodicals, Inc.
Conflict of interest statement
Conflicts of interest: The authors of this manuscript have no disclosures or conflicts of interest to report. No competing financial interests exists.
Figures



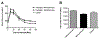



Similar articles
-
Evaluation of encapsulating and microporous nondegradable hydrogel scaffold designs on islet engraftment in rodent models of diabetes.Biotechnol Bioeng. 2018 Sep;115(9):2356-2364. doi: 10.1002/bit.26741. Epub 2018 Jun 25. Biotechnol Bioeng. 2018. PMID: 29873059 Free PMC article.
-
Design of a vascularized synthetic poly(ethylene glycol) macroencapsulation device for islet transplantation.Biomaterials. 2018 Jul;172:54-65. doi: 10.1016/j.biomaterials.2018.04.047. Epub 2018 Apr 25. Biomaterials. 2018. PMID: 29715595 Free PMC article.
-
Immunoisolation of murine islet allografts in vascularized sites through conformal coating with polyethylene glycol.Am J Transplant. 2018 Mar;18(3):590-603. doi: 10.1111/ajt.14547. Epub 2017 Dec 2. Am J Transplant. 2018. PMID: 29068143 Free PMC article.
-
Multifunctional Islet Transplantation Hydrogel Encapsulating A20 High-Expressing Islets.Drug Des Devel Ther. 2020 Sep 29;14:4021-4027. doi: 10.2147/DDDT.S273050. eCollection 2020. Drug Des Devel Ther. 2020. PMID: 33061306 Free PMC article. Review.
-
Emerging approaches for the development of artificial islets.Smart Med. 2024 Mar 7;3(2):e20230042. doi: 10.1002/SMMD.20230042. eCollection 2024 Jun. Smart Med. 2024. PMID: 39188698 Free PMC article. Review.
Cited by
-
In vivo reprogramming of immune cells: Technologies for induction of antigen-specific tolerance.Adv Drug Deliv Rev. 2017 May 15;114:240-255. doi: 10.1016/j.addr.2017.04.005. Epub 2017 Apr 14. Adv Drug Deliv Rev. 2017. PMID: 28414079 Free PMC article. Review.
-
Evaluation of encapsulating and microporous nondegradable hydrogel scaffold designs on islet engraftment in rodent models of diabetes.Biotechnol Bioeng. 2018 Sep;115(9):2356-2364. doi: 10.1002/bit.26741. Epub 2018 Jun 25. Biotechnol Bioeng. 2018. PMID: 29873059 Free PMC article.
-
Modulating the foreign body response of implants for diabetes treatment.Adv Drug Deliv Rev. 2021 Jul;174:87-113. doi: 10.1016/j.addr.2021.01.011. Epub 2021 Jan 21. Adv Drug Deliv Rev. 2021. PMID: 33484736 Free PMC article. Review.
-
It's All in the Delivery: Designing Hydrogels for Cell and Non-viral Gene Therapies.Mol Ther. 2018 Sep 5;26(9):2087-2106. doi: 10.1016/j.ymthe.2018.07.022. Epub 2018 Aug 4. Mol Ther. 2018. PMID: 30107997 Free PMC article. Review.
-
The emerging field of pancreatic tissue engineering: A systematic review and evidence map of scaffold materials and scaffolding techniques for insulin-secreting cells.J Tissue Eng. 2019 Oct 30;10:2041731419884708. doi: 10.1177/2041731419884708. eCollection 2019 Jan-Dec. J Tissue Eng. 2019. PMID: 31700597 Free PMC article. Review.
References
-
- Beck J, Angus R, Madsen B, Britt D, Vernon B, Nguyen KT. 2007. Islet encapsulation: Strategies to enhance islet cell functions. Tissue Eng 13:589–599. http://www.ncbi.nlm.nih.gov/pubmed/17518605 - PubMed
-
- Blomeier H, Zhang X, Rives C, Brissova M, Hughes E, Baker M, Powers AC, Kaufman DB, Shea LD, Lowe WL. 2006. Polymer scaffolds as synthetic microenvironments for extrahepatic islet transplantation. Transplantation 82:452–459. http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=2648394&tool=p... - PMC - PubMed
-
- Buder B, Alexander M, Krishnan R, Chapman DW, Lakey JR. 2013. Encapsulated islet transplantation: Strategies and clinical trials. Immune Netw 13:235–239. http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=3875781&tool=p... - PMC - PubMed
-
- Chiu Y-C, Cheng M-H, Engel H, Kao S-W, Larson JC, Gupta S, Brey EM. 2011. The role of pore size on vascularization and tissue remodeling in PEG hydrogels. Biomaterials 32:6045–6051. http://www.ncbi.nlm.nih.gov/pubmed/21663958 - PubMed
-
- Colton CK. 2014. Oxygen supply to encapsulated therapeutic cells. Adv Drug Deliv Rev 68:93–110. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

