Microsomal Prostaglandin E Synthase-1 Expression by Aortic Smooth Muscle Cells Attenuates the Differentiated Phenotype
- PMID: 27159620
- PMCID: PMC4980264
- DOI: 10.1097/FJC.0000000000000395
Microsomal Prostaglandin E Synthase-1 Expression by Aortic Smooth Muscle Cells Attenuates the Differentiated Phenotype
Abstract
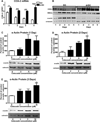
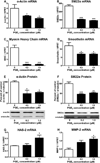
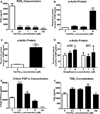
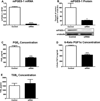
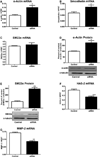
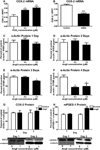
The development of numerous types of cardiovascular disease is associated with alteration of the vascular smooth muscle cell (SMC) phenotype. We have previously shown that abdominal aortic aneurysm progression in a mouse model of the disease is associated with reduced differentiation of SMCs within the lesion and that cyclooxygenase-2 (COX-2) is critical to initiation and progression of the aneurysms. The current studies used human aortic SMC (hASMC) cultures to better characterize mechanisms responsible for COX-2-dependent modulation of the SMC phenotype. Depending on the culture conditions, hASMCs expressed multiple characteristics of a differentiated and contractile phenotype, or a dedifferentiated and secretory phenotype. The pharmacological inhibition of COX-2 promoted the differentiated phenotype, whereas treatment with the COX-2-derived metabolite prostaglandin E2 (PGE2) increased characteristics of the dedifferentiated phenotype. Furthermore, pharmacological inhibition or siRNA-mediated knockdown of microsomal prostaglandin E synthase-1 (mPGES-1), the enzyme that functions downstream of COX-2 during the synthesis of PGE2, significantly increased expression of characteristics of the differentiated SMC phenotype. Therefore, our findings suggest that COX-2 and mPGES-1-dependent synthesis of PGE2 contributes to a dedifferentiated hASMC phenotype and that mPGES-1 may provide a novel pharmacological target for treatment of cardiovascular diseases where altered SMC differentiation has a causative role.
Conflict of interest statement
The authors have no conflicts of interest to disclose.
Figures

References
-
- Owens GK, Kumar MS, Wamhoff BR. Molecular regulation of vascular smooth muscle cell differentiation in development and disease. Physiol Rev. 2004 Jul;84(3):767–801. - PubMed
-
- Rzucidlo EM, Martin KA, Powell RJ. Regulation of vascular smooth muscle cell differentiation. J Vasc Surg. 2007 Jun;(45 Suppl A):A25–A32. - PubMed
-
- Roan JN, Tsai YC, Chen IW, Chang SW, Huang CC, Lam CF. Inhibition of cyclooxygenase-2 modulates phenotypic switching of vascular smooth muscle cells during increased aortic blood flow. Heart and vessels. 2012 May;27(3):307–315. - PubMed
-
- Zhu L, Vranckx R, Khau Van Kien P, Lalande A, Boisset N, Mathieu F, Wegman M, Glancy L, Gasc JM, Brunotte F, Bruneval P, Wolf JE, Michel JB, Jeunemaitre X. Mutations in myosin heavy chain 11 cause a syndrome associating thoracic aortic aneurysm/aortic dissection and patent ductus arteriosus. Nat Genet. 2006 Mar;38(3):343–349. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

