Sex and Gender Differences in Risk, Pathophysiology and Complications of Type 2 Diabetes Mellitus
- PMID: 27159875
- PMCID: PMC4890267
- DOI: 10.1210/er.2015-1137
Sex and Gender Differences in Risk, Pathophysiology and Complications of Type 2 Diabetes Mellitus
Abstract
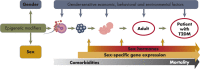
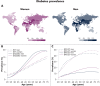
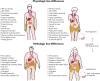
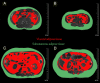
The steep rise of type 2 diabetes mellitus (T2DM) and associated complications go along with mounting evidence of clinically important sex and gender differences. T2DM is more frequently diagnosed at lower age and body mass index in men; however, the most prominent risk factor, which is obesity, is more common in women. Generally, large sex-ratio differences across countries are observed. Diversities in biology, culture, lifestyle, environment, and socioeconomic status impact differences between males and females in predisposition, development, and clinical presentation. Genetic effects and epigenetic mechanisms, nutritional factors and sedentary lifestyle affect risk and complications differently in both sexes. Furthermore, sex hormones have a great impact on energy metabolism, body composition, vascular function, and inflammatory responses. Thus, endocrine imbalances relate to unfavorable cardiometabolic traits, observable in women with androgen excess or men with hypogonadism. Both biological and psychosocial factors are responsible for sex and gender differences in diabetes risk and outcome. Overall, psychosocial stress appears to have greater impact on women rather than on men. In addition, women have greater increases of cardiovascular risk, myocardial infarction, and stroke mortality than men, compared with nondiabetic subjects. However, when dialysis therapy is initiated, mortality is comparable in both males and females. Diabetes appears to attenuate the protective effect of the female sex in the development of cardiac diseases and nephropathy. Endocrine and behavioral factors are involved in gender inequalities and affect the outcome. More research regarding sex-dimorphic pathophysiological mechanisms of T2DM and its complications could contribute to more personalized diabetes care in the future and would thus promote more awareness in terms of sex- and gender-specific risk factors.
Figures

References
-
- Schiebinger L, Klinge I, Sánchez de Madariaga I, Paik H, Schraudner M, Stefanick M. Gendered innovations in science, health, medicine, engineering and environment. 2011–2015. Available at http://genderedinnovations.stanford.edu/ Accessed January 10, 2015.
-
- EUGenMed Cardiovascular Clinical Study Group, Regitz-Zagrosek V, Oertelt-Prigione S, et al. Gender in cardiovascular diseases: impact on clinical manifestations, management, and outcomes. Eur Heart J. 2016;37:24–34. - PubMed
-
- Annandale E, Riska E. New connections: towards a gender-inclusive approach to women's and men's health. Curr Sociol. 2009;57:123–133.
-
- Geary N. Counterpoint: physiologists should not distinguish “sex” and “gender.” Am J Physiol Regul Integr Comp Physiol. 2010;298:R1702–R1704. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

