Creation of zebularine-resistant human cytidine deaminase mutants to enhance the chemoprotection of hematopoietic stem cells
- PMID: 27160178
- PMCID: PMC5181380
- DOI: 10.1093/protein/gzw012
Creation of zebularine-resistant human cytidine deaminase mutants to enhance the chemoprotection of hematopoietic stem cells
Abstract
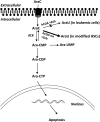
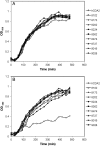
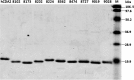
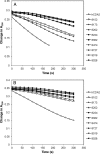
Human cytidine deaminase (hCDA) is a biomedically important enzyme able to inactivate cytidine nucleoside analogs such as the antileukemic agent cytosine arabinoside (AraC) and thereby limit antineoplastic efficacy. Potent inhibitors of hCDA have been developed, e.g. zebularine, that when administered in combination with AraC enhance antineoplastic activity. Tandem hematopoietic stem cell (HSC) transplantation and combination chemotherapy (zebularine and AraC) could exhibit robust antineoplastic potency, but AraC-based chemotherapy regimens lead to pronounced myelosuppression due to relatively low hCDA activity in HSCs, and this approach could exacerbate this effect. To circumvent the pronounced myelosuppression of zebularine and AraC combination therapy while maintaining antineoplastic potency, zebularine-resistant hCDA variants could be used to gene-modify HSCs prior to transplantation. To achieve this, our approach was to isolate hCDA variants through random mutagenesis in conjunction with selection for hCDA activity and resistance to zebularine in an Escherichia coli genetic complementation system. Here, we report the identification of nine novel variants from a pool of 1.6 × 106 transformants that conferred significant zebularine resistance relative to wild-type hCDA2. Several variants revealed significantly higher Ki values toward zebularine when compared with wild-type hCDA values and, as such, are candidates for further exploration for gene-modified HSC transplantation approaches.
Keywords: cytidine deaminase; cytosine arabinoside; hematopoietic stem cells; random mutagenesis; zebularine.
© The Author 2016. Published by Oxford University Press. All rights reserved. For Permissions, please e-mail: journals.permissions@oup.com.
Figures



Similar articles
-
Inhibition of cytidine deaminase by zebularine enhances the antineoplastic action of 5-aza-2'-deoxycytidine.Cancer Chemother Pharmacol. 2009 Feb;63(3):411-6. doi: 10.1007/s00280-008-0750-6. Epub 2008 Apr 9. Cancer Chemother Pharmacol. 2009. PMID: 18398609
-
Potent inhibitors for the deamination of cytosine arabinoside and 5-aza-2'-deoxycytidine by human cytidine deaminase.Cancer Chemother Pharmacol. 1992;30(1):7-11. doi: 10.1007/BF00686478. Cancer Chemother Pharmacol. 1992. PMID: 1375134
-
Selection of drug-resistant transduced cells with cytosine nucleoside analogs using the human cytidine deaminase gene.Cancer Gene Ther. 2001 Sep;8(9):669-76. doi: 10.1038/sj.cgt.7700358. Cancer Gene Ther. 2001. PMID: 11593336
-
Zebularine: a unique molecule for an epigenetically based strategy in cancer chemotherapy. The magic of its chemistry and biology.Nucleosides Nucleotides Nucleic Acids. 2005;24(5-7):305-18. doi: 10.1081/ncn-200059765. Nucleosides Nucleotides Nucleic Acids. 2005. PMID: 16247946 Review.
-
Zebularine: a unique molecule for an epigenetically based strategy in cancer chemotherapy.Ann N Y Acad Sci. 2005 Nov;1058:246-54. doi: 10.1196/annals.1359.037. Ann N Y Acad Sci. 2005. PMID: 16394141 Review.
Cited by
-
Zebularine Promotes Hepatic Differentiation of Rabbit Bone Marrow Mesenchymal Stem Cells by Interfering with p38 MAPK Signaling.Stem Cells Int. 2018 Oct 10;2018:9612512. doi: 10.1155/2018/9612512. eCollection 2018. Stem Cells Int. 2018. PMID: 30405726 Free PMC article.
References
-
- Bardenheuer W., Lehmberg K., Rattmann I., Brueckner A., Schneider A., Sorg U.R., Seeber S., Moritz T., Flasshove M. (2005) Leukemia, 19, 2281–2288. - PubMed
-
- Barrios N.J., Tebbi C.K., Freeman A.I., Brecher M.L. (1987) Cancer, 60, 165–169. - PubMed
-
- Beauséjour C.M., Eliopoulos N., Momparler L., Le N.L., Momparler R.L. (2001) Cancer Gene Ther., 8, 669–676. - PubMed
-
- Betts L., Xiang S., Short S.A., Wolfenden R., Carter C.W. Jr (1994) J. Mol. Biol., 235, 635–656. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

