Nephrotoxicity after PRRT with (177)Lu-DOTA-octreotate
- PMID: 27160225
- PMCID: PMC4969358
- DOI: 10.1007/s00259-016-3382-9
Nephrotoxicity after PRRT with (177)Lu-DOTA-octreotate
Abstract
Purpose: After peptide receptor radionuclide therapy (PRRT), renal toxicity may occur, particular in PRRT with (90)Y-labelled somatostatin analogues. Risk factors have been identified for increased probability of developing renal toxicity after PRRT, including hypertension, diabetes and age. We investigated the renal function over time, the incidence of nephrotoxicity and associated risk factors in patients treated with PRRT with [(177)Lu-DOTA(0),Tyr(3)]-Octreotate ((177)Lu-Octreotate). Also, radiation dose to the kidneys was evaluated and compared with the accepted dose limits in external beam radiotherapy and PRRT with (90)Y-radiolabelled somatostatin analogues.
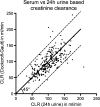
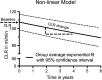
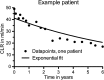
Methods: The annual decrease in creatinine clearance (CLR) was determined in 209 Dutch patients and the incidence of grade 3 or 4 renal toxicity (according to CTCAE v4.03) was evaluated in 323 patients. Risk factors were analysed using a nonlinear mixed effects regression model. Also, radiation doses to the kidneys were calculated and their association with high annual decrease in renal function were analysed.
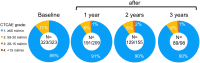
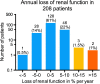
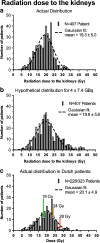
Results: Of the 323 patients, 3 (1 %) developed (subacute) renal toxicity grade 2 (increase in serum creatinine >1.5 - 3.0 times baseline or upper limit of normal). No subacute grade 3 or 4 nephrotoxicity was observed. The estimated average baseline CLR (± SD) was 108 ± 5 ml/min and the estimated average annual decrease in CLR (± SD) was 3.4 ± 0.4 %. None of the risk factors (hypertension, diabetes, high cumulative injected activity, radiation dose to the kidneys and CTCAE grade) at baseline had a significant effect on renal function over time. The mean absorbed kidney dose in 228 patients was 20.1 ± 4.9 Gy.
Conclusion: Nephrotoxicity in patients treated with (177)Lu-octreotate was low. No (sub)acute grade 3 or 4 renal toxicity occurred and none of the patients had an annual decrease in renal function of >20 %. No risk factors for renal toxicity could be identified. Our data support the idea that the radiation dose threshold, adopted from external beam radiotherapy and PRRT with (90)Y-labelled somatostatin analogues, does not seem valid for PRRT with (177)Lu-octreotate.
Keywords: 177Lu-Octreotate; Dosimetry; Kidneys; Nephrotoxicity; PRRT; Renal function; Toxicity.
Figures
References
-
- Valkema R, Pauwels SA, Kvols LK, Kwekkeboom DJ, Jamar F, de Jong M, et al. Long-term follow-up of renal function after peptide receptor radiation therapy with (90)Y-DOTA(0), Tyr(3)-octreotide and (177)Lu-DOTA(0), Tyr(3)-octreotate. J Nucl Med. 2005;46(Suppl 1):83S–91S. - PubMed
-
- Bodei L, Cremonesi M, Ferrari M, Pacifici M, Grana CM, Bartolomei M, et al. Long-term evaluation of renal toxicity after peptide receptor radionuclide therapy with 90Y-DOTATOC and 177Lu-DOTATATE: the role of associated risk factors. Eur J Nucl Med Mol Imaging. 2008;35:1847–1856. doi: 10.1007/s00259-008-0778-1. - DOI - PubMed
-
- Imhof A, Brunner P, Marincek N, Briel M, Schindler C, Rasch H, et al. Response, survival, and long-term toxicity after therapy with the radiolabeled somatostatin analogue [90Y-DOTA]-TOC in metastasized neuroendocrine cancers. J Clin Oncol. 2011;29:2416–2423. doi: 10.1200/JCO.2010.33.7873. - DOI - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

