Evaluation of the Cochrane tool for assessing risk of bias in randomized clinical trials: overview of published comments and analysis of user practice in Cochrane and non-Cochrane reviews
- PMID: 27160280
- PMCID: PMC4862216
- DOI: 10.1186/s13643-016-0259-8
Evaluation of the Cochrane tool for assessing risk of bias in randomized clinical trials: overview of published comments and analysis of user practice in Cochrane and non-Cochrane reviews
Abstract
Background: The Cochrane risk of bias tool for randomized clinical trials was introduced in 2008 and has frequently been commented on and used in systematic reviews. We wanted to evaluate the tool by reviewing published comments on its strengths and challenges and by describing and analysing how the tool is applied to both Cochrane and non-Cochrane systematic reviews.
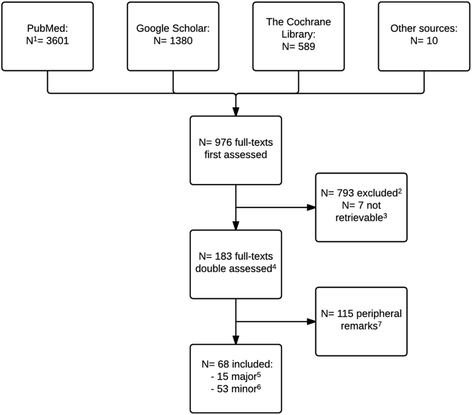
Methods: A review of published comments (searches in PubMed, The Cochrane Methodology Register and Google Scholar) and an observational study (100 Cochrane and 100 non-Cochrane reviews from 2014).
Results: Our review included 68 comments, 15 of which were categorised as major. The main strengths of the tool were considered to be its aim (to assess trial conduct and not reporting), its developmental basis (wide consultation, empirical and theoretical evidence) and its transparent procedures. The challenges of the tool were mainly considered to be its choice of core bias domains (e.g. not involving funding/conflicts of interest) and issues to do with implementation (i.e. modest inter-rater agreement) and terminology. Our observational study found that the tool was used in all Cochrane reviews (100/100) and was the preferred tool in non-Cochrane reviews (31/100). Both types of reviews frequently implemented the tool in non-recommended ways. Most Cochrane reviews planned to use risk of bias assessments as basis for sensitivity analyses (70 %), but only a minority conducted such analyses (19 %) because, in many cases, few trials were assessed as having "low" risk of bias for all standard domains (6 %). The judgement of at least one risk of bias domain as "unclear" was found in 89 % of included randomized clinical trials (1103/1242).
Conclusions: The Cochrane tool has become the standard approach to assess risk of bias in randomized clinical trials but is frequently implemented in a non-recommended way. Based on published comments and how it is applied in practice in systematic reviews, the tool may be further improved by a revised structure and more focused guidance.
Keywords: Bias; Cochrane; Comment; Randomized clinical trial; Systematic review; Tool; User practice.
Figures
References
-
- Lundh A, Sismondo S, Lexchin J, Busuioc OA, Bero L. Industry sponsorship and research outcome. Cochrane Database Syst Rev. 2012;12:MR000033. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

