Synthesis of 2-Aryl- and 2-Vinylpyrrolidines via Copper-Catalyzed Coupling of Styrenes and Dienes with Potassium β-Aminoethyl Trifluoroborates
- PMID: 27160334
- PMCID: PMC4874882
- DOI: 10.1021/acs.orglett.6b01259
Synthesis of 2-Aryl- and 2-Vinylpyrrolidines via Copper-Catalyzed Coupling of Styrenes and Dienes with Potassium β-Aminoethyl Trifluoroborates
Abstract
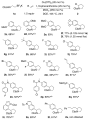
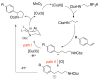
2-Arylpyrrolidines occur frequently in bioactive compounds, and thus, methods to access them from readily available reagents are valuable. We report a copper-catalyzed intermolecular carboamination of vinylarenes with potassium N-carbamoyl-β-aminoethyltrifluoroborates. The reaction occurs with terminal, 1,2-disubstituted, and 1,1-disubstituted vinylarenes bearing a number of functional groups. 1,3-Dienes are also good substrates, and their reactions give 2-vinylpyrrolidines. Radical clock mechanistic experiments are consistent with the presence of carbon radical intermediates and do not support participation of carbocations.
Figures





References
-
- Klapars A, Campos KR, Waldman JH, Zewge D, Domer PG, Chen C-Y. J. Org. Chem. 2008;73:4986. - PubMed
-
- Cho-Schultz S, Patten MJ, Huang B, Elleraas J, Gajiwala KS, Hickey MJ, Wang J, Mehta PP, Kang P, Gehring MR, Kung P-P, Sutton SC. J. Comb. Chem. 2009;11:860. - PubMed
-
- Elliott RL, Ryther KB, Anderson DJ, Raszkiewicz JL, Campbell JE, Sullivan JP, Garvey DS. Bioorg. Med. Chem. Lett. 1995;5:991.
-
- Wagner FF, Comins DL. Tetrahedron. 2007;63:8065.
-
- Campos KR, Klapars A, Waldman JH, Dormer PG, Chen CY. J. Am. Chem. Soc. 2006;128:3538. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

