Efficacy and safety of cannabinoid oromucosal spray for multiple sclerosis spasticity
- PMID: 27160523
- PMCID: PMC5013116
- DOI: 10.1136/jnnp-2015-312591
Efficacy and safety of cannabinoid oromucosal spray for multiple sclerosis spasticity
Abstract
Background: The approval of 9-δ-tetrahydocannabinol and cannabidiol (THC:CBD) oromucosal spray (Sativex) for the management of treatment-resistant multiple sclerosis (MS) spasticity opened a new opportunity for many patients. The aim of our study was to describe Sativex effectiveness and adverse events profile in a large population of Italian patients with MS in the daily practice setting.
Methods: We collected data of all patients starting Sativex between January 2014 and February 2015 from the mandatory Italian medicines agency (AIFA) e-registry. Spasticity assessment by the 0-10 numerical rating scale (NRS) scale is available at baseline, after 1 month of treatment (trial period), and at 3 and 6 months.
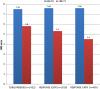
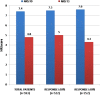
Results: A total of 1615 patients were recruited from 30 MS centres across Italy. After one treatment month (trial period), we found 70.5% of patients reaching a ≥20% improvement (initial response, IR) and 28.2% who had already reached a ≥30% improvement (clinically relevant response, CRR), with a mean NRS score reduction of 22.6% (from 7.5 to 5.8). After a multivariate analysis, we found an increased probability to reach IR at the first month among patients with primary and secondary progressive MS, (n=1169, OR 1.4 95% CI 1.04 to 1.9, p=0.025) and among patients with >8 NRS score at baseline (OR 1.8 95% CI 1.3-2.4 p<0.001). During the 6 months observation period, 631(39.5%) patients discontinued treatment. The main reasons for discontinuation were lack of effectiveness (n=375, 26.2%) and/or adverse events (n=268, 18.7%).
Conclusions: Sativex can be a useful and safe option for patients with MS with moderate to severe spasticity resistant to common antispastic drugs.
Published by the BMJ Publishing Group Limited. For permission to use (where not already granted under a licence) please go to http://www.bmj.com/company/products-services/rights-and-licensing/
Figures
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical