How Often Does Neoadjuvant Chemotherapy Avoid Axillary Dissection in Patients With Histologically Confirmed Nodal Metastases? Results of a Prospective Study
- PMID: 27160528
- PMCID: PMC5070651
- DOI: 10.1245/s10434-016-5246-8
How Often Does Neoadjuvant Chemotherapy Avoid Axillary Dissection in Patients With Histologically Confirmed Nodal Metastases? Results of a Prospective Study
Abstract
Background: In breast cancer patients with nodal metastases at presentation, false-negative rates lower than 10 % have been demonstrated for sentinel node biopsy (SLNB) after neoadjuvant chemotherapy (NAC) when three or more negative sentinel nodes (SLNs) are retrieved. However, the frequency with which axillary dissection (ALND) can be avoided is uncertain.
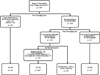
Methods: Among 534 prospectively identified consecutive patients with clinical stages 2 and 3 cancer receiving NAC from November 2013 to November 2015, all biopsy-proven node-positive (N+) cases were identified. Patients clinically node-negative after NAC were eligible for SLNB. The indications for ALND were failed mapping, fewer than three SLNs retrieved, and positive SLNs.
Results: Of 288 N+ patients, 195 completed surgery, with 132 (68 %) of these patients eligible for SLNB. The median age was 50 years. Of these patients, 73 (55 %) were estrogen receptor-positive (ER+), 21 (16 %) were ER- and human epidermal growth factor receptor-2-positive (HER2+), and 38 (29 %) were triple-negative. In four cases, SLNB was deferred intraoperatively. Among 128 SLNB attempts, three or more SLNs were retrieved in 110 cases (86 %), one or two SLNs were retrieved in 15 cases (12 %), and failed mapping occurred in three cases (2 %). In 66 cases, ALND was indicated: 54 (82 %) for positive SLNs, 9 (14 %) for fewer than three negative SLNs, and 3 (4 %) for failed mapping. Persistent disease was found in 17 % of the patients with fewer than three negative SLNs retrieved. Of the 128 SLNB cases, 62 (48 %) had SLNB alone with three or more SLNs retrieved. Among 195 N+ patients who completed surgery, nodal pathologic complete response (pCR) was achieved for 49 %, with rates ranging from 21 % for ER+/HER2- to 97 % for ER-/HER2+ cases, and was significantly more common than breast pCR in ER+/HER2- and triple-negative cases.
Conclusions: Nearly 70 % of the N+ patients were eligible for SLNB after NAC. For 48 %, ALND was avoided, supporting the role of NAC in reducing the need for ALND among patients presenting with nodal metastases.
Conflict of interest statement
The authors have no conflicts of interest to declare
Figures

Comment in
-
Sentinel Lymph Node Biopsy after Neoadjuvant Chemotherapy for Patients with Axillary Metastases: Can We Avoid the Unavoidable?Ann Surg Oncol. 2016 Oct;23(11):3429-3431. doi: 10.1245/s10434-016-5455-1. Epub 2016 Jul 26. Ann Surg Oncol. 2016. PMID: 27459978 No abstract available.
References
-
- King TA, Morrow M. Surgical issues in patients with breast cancer receiving neoadjuvant chemotherapy. Nat Rev Clin Oncol. 2015;12(6):335–343. - PubMed
-
- Xing Y, Foy M, Cox DD, Kuerer HM, Hunt KK, Cormier JN. Meta-analysis of sentinel lymph node biopsy after preoperative chemotherapy in patients with breast cancer. Br J Surg. 2006;93(5):539–546. - PubMed
-
- Kelly AM, Dwamena B, Cronin P, Carlos RC. Breast cancer sentinel node identification and classification after neoadjuvant chemotherapy-systematic review and meta analysis. Acad Radiol. 2009;16(5):551–563. - PubMed
-
- Kuehn T, Bauerfeind I, Fehm T, Fleige B, Hausschild M, Helms G, et al. Sentinel-lymph-node biopsy in patients with breast cancer before and after neoadjuvant chemotherapy (SENTINA): A prospective, multicentre cohort study. Lancet Oncol. 2013;14(7):609–618. - PubMed
-
- Boileau JF, Poirier B, Basik M, Holloway CMB, Gaboury L, Sideris L, et al. Sentinel node biopsy after neoadjuvant chemotherapy in biopsy-proven node-positive breast cancer: The SN FNAC study. J Clin Oncol. 2015;33(3):258–263. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

