Phase 2 Study of the Safety and Antitumor Activity of Apalutamide (ARN-509), a Potent Androgen Receptor Antagonist, in the High-risk Nonmetastatic Castration-resistant Prostate Cancer Cohort
- PMID: 27160947
- PMCID: PMC5568792
- DOI: 10.1016/j.eururo.2016.04.023
Phase 2 Study of the Safety and Antitumor Activity of Apalutamide (ARN-509), a Potent Androgen Receptor Antagonist, in the High-risk Nonmetastatic Castration-resistant Prostate Cancer Cohort
Abstract
Background: Apalutamide is a potent androgen receptor (AR) antagonist that targets the AR ligand-binding domain and prevents AR nuclear translocation, DNA binding, and transcription of AR gene targets.
Objective: To evaluate the activity and safety of apalutamide in patients with high-risk nonmetastatic castration-resistant prostate cancer (nmCRPC).
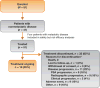
Design, setting, and participants: We conducted a multicenter phase 2 study of nmCRPC patients with a high risk for progression (prostate-specific antigen [PSA] ≥8 ng/ml or PSA doubling time [PSA DT] ≤10 mo).
Intervention: Patients received 240mg/d apalutamide while continuing on androgen-deprivation therapy.
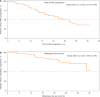
Outcome measurements and statistical analysis: Primary end point was 12-wk PSA response (Prostate Cancer Working Group 2 criteria). Secondary end points included safety, time to PSA progression (TTPP), and metastasis-free survival (MFS).
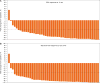
Results and limitations: A total of 51 patients were enrolled; four patients with metastatic disease were excluded from the efficacy analysis. Patient characteristics included median age, 71 yr; Eastern Cooperative Oncology Group performance status 0 (76%); Gleason score ≤7 (57%); median PSA 10.7 ng/ml; and PSA DT ≤10 mo (45%). At median follow-up of 28.0 mo, 18 patients (35%) remained in the study. Overall, 89% of patients had ≥50% PSA decline at 12 wk. Median TTPP was 24.0 mo (95% confidence interval [CI], 16.3 mo-not reached [NR]); median MFS was NR (95% CI, 33.4 mo-NR). Most of the patients discontinued study treatment (n=33) due to disease progression (n=11 [22%]) or adverse events (AEs) (n=9 [18%]). The most common AE was fatigue (any grade, n=31 [61%]) although grade ≥3 fatigue was uncommon (n=2 [4%]). These represent the first apalutamide nmCRPC patient clinical data.
Conclusions: In high-risk nmCRPC patients, apalutamide was safe with robust activity based on durable PSA responses and disease control.
Patient summary: Antitumor activity and the safety of apalutamide in patients with nonmetastatic castration-resistant prostate cancer support continued development in this setting.
Trial registration: ClinicalTrials.gov identifier NCT01171898.
Keywords: Antitumor activity; Apalutamide; Castration-resistant prostate cancer; Safety.
Copyright © 2016 European Association of Urology. Published by Elsevier B.V. All rights reserved.
Conflict of interest statement
Figures

Comment in
-
Second-generation Androgen Receptor-targeted Therapies in Nonmetastatic Castration-resistant Prostate Cancer: Effective Early Intervention or Intervening Too Early?Eur Urol. 2016 Dec;70(6):971-973. doi: 10.1016/j.eururo.2016.05.026. Epub 2016 May 26. Eur Urol. 2016. PMID: 27238654 No abstract available.
References
-
- Chi KN, Bjartell A, Dearnaley D, et al. Castration-resistant prostate cancer: from new pathophysiology to new treatment targets. Eur Urol. 2009;56:594–605. - PubMed
-
- Smith MR, Kabbinavar F, Saad F, et al. Natural history of rising serum prostate-specific antigen in men with castrate nonmetastatic prostate cancer. J Clin Oncol. 2005;23:2918–25. - PubMed
-
- Prostate cancer, v.1.2016. National Comprehensive Cancer Network; NCCN clinical practice guidelines in oncology (NCCN Guidelines) Web site. http://www.nccn.org/professionals/physician_gls/pdf/prostate.pdf. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

