Extracellular microRNAs and endothelial hyperglycaemic memory: a therapeutic opportunity?
- PMID: 27161301
- PMCID: PMC5094499
- DOI: 10.1111/dom.12688
Extracellular microRNAs and endothelial hyperglycaemic memory: a therapeutic opportunity?
Abstract
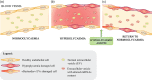
Type 2 diabetes mellitus (T2DM) is a major cause of cardiovascular (CV) disease. Several large clinical trials have shown that the risk for patients with diabetes of developing CV complications is only partially reduced by early, intensive glycaemic control and lifestyle interventions, and that such complications result from changes in complex, not fully explored networks that contribute to the maintenance of endothelial function. The accumulation of senescent cells and the low-grade, systemic, inflammatory status that accompanies aging (inflammaging) are involved in the development of endothelial dysfunction. Such phenomena are modulated by epigenetic mechanisms, including microRNAs (miRNAs). MiRNAs can modulate virtually all gene transcripts. They can be secreted by living cells and taken up in active form by recipient cells, providing a new communication tool between tissues and organs. MiRNA deregulation has been associated with the development and progression of a number of age-related diseases, including the enduring gene expression changes seen in patients with diabetes. We review recent evidence on miRNA changes in T2DM, focusing on the ability of diabetes-associated miRNAs to modulate endothelial function, inflammaging and cellular senescence. We also discuss the hypothesis that miRNA-containing extracellular vesicles (i.e. exosomes and microvesicles) could be harnessed to restore a 'physiological' signature capable of preventing or delaying the harmful systemic effects of T2DM.
Keywords: antidiabetic drug; cardiovascular disease; diabetes complications; extracellular vesicles, exosomes; glycaemic control; metabolic memory; metformin; microRNAs; type 2 diabetes.
© 2016 The Authors. Diabetes, Obesity and Metabolism published by John Wiley & Sons Ltd.
Figures

References
-
- Ceriello A. The emerging challenge in diabetes: the "metabolic memory". Vascul Pharmacol 2012; 57: 133–138. - PubMed
-
- Laakso M, Kuusisto J. Insulin resistance and hyperglycaemia in cardiovascular disease development. Nat Rev Endocrinol 2014; 10: 293–302. - PubMed
-
- Stehouwer CD, Gall MA, Twisk JW, Knudsen E, Emeis JJ, Parving HH. Increased urinary albumin excretion, endothelial dysfunction, and chronic low‐grade inflammation in type 2 diabetes: progressive, interrelated, and independently associated with risk of death. Diabetes 2002; 51: 1157–1165. - PubMed
-
- Guarner V, Rubio‐Ruiz ME. Low‐grade systemic inflammation connects aging, metabolic syndrome and cardiovascular disease. Interdiscip Top Gerontol 2015; 40: 99–106. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

