In vivo physiological recording from the lateral line of juvenile zebrafish
- PMID: 27161862
- PMCID: PMC5043028
- DOI: 10.1113/JP271794
In vivo physiological recording from the lateral line of juvenile zebrafish
Abstract
Key points: Zebrafish provide a unique opportunity to investigate in vivo sensory transduction in mature hair cells. We have developed a method for studying the biophysical properties of mature hair cells from the lateral line of juvenile zebrafish. The method involves application of the anaesthetic benzocaine and intubation to maintain ventilation and oxygenation through the gills. The same approach could be used for in vivo functional studies in other sensory and non-sensory systems from juvenile and adult zebrafish.
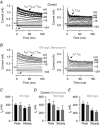
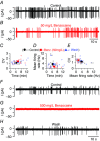
Abstract: Hair cells are sensory receptors responsible for transducing auditory and vestibular information into electrical signals, which are then transmitted with remarkable precision to afferent neurons. The zebrafish lateral line is emerging as an excellent in vivo model for genetic and physiological analysis of hair cells and neurons. However, research has been limited to larval stages because zebrafish become protected from the time of independent feeding under European law (from 5.2 days post-fertilization (dpf) at 28.5°C). In larval zebrafish, the functional properties of most of hair cells, as well as those of other excitable cells, are still immature. We have developed an experimental protocol to record electrophysiological properties from hair cells of the lateral line in juvenile zebrafish. We found that the anaesthetic benzocaine at 50 mg l(-1) was an effective and safe anaesthetic to use on juvenile zebrafish. Concentrations up to 300 mg l(-1) did not affect the electrical properties or synaptic vesicle release of juvenile hair cells, unlike the commonly used anaesthetic MS-222, which reduces the size of basolateral membrane K(+) currents. Additionally, we implemented a method to maintain gill movement, and as such respiration and blood oxygenation, via the intubation of > 21 dpf zebrafish. The combination of benzocaine and intubation provides an experimental platform to investigate the physiology of mature hair cells from live zebrafish. More generally, this method would allow functional studies involving live imaging and electrophysiology from juvenile and adult zebrafish.
© 2016 The Authors. The Journal of Physiology published by John Wiley & Sons Ltd on behalf of The Physiological Society.
Figures




Similar articles
-
In vivo and in vitro biophysical properties of hair cells from the lateral line and inner ear of developing and adult zebrafish.J Physiol. 2014 May 15;592(10):2041-58. doi: 10.1113/jphysiol.2013.265108. Epub 2014 Feb 24. J Physiol. 2014. PMID: 24566541 Free PMC article.
-
Functional development and regeneration of hair cells in the zebrafish lateral line.J Physiol. 2021 Aug;599(16):3913-3936. doi: 10.1113/JP281522. Epub 2021 Jul 9. J Physiol. 2021. PMID: 34143497 Free PMC article.
-
Physiological recordings from the zebrafish lateral line.Methods Cell Biol. 2016;133:253-79. doi: 10.1016/bs.mcb.2016.02.004. Epub 2016 Mar 4. Methods Cell Biol. 2016. PMID: 27263416
-
There and back again: development and regeneration of the zebrafish lateral line system.Wiley Interdiscip Rev Dev Biol. 2015 Jan-Feb;4(1):1-16. doi: 10.1002/wdev.160. Epub 2014 Oct 20. Wiley Interdiscip Rev Dev Biol. 2015. PMID: 25330982 Free PMC article. Review.
-
Behavior, Electrophysiology, and Robotics Experiments to Study Lateral Line Sensing in Fishes.Integr Comp Biol. 2018 Nov 1;58(5):874-883. doi: 10.1093/icb/icy066. Integr Comp Biol. 2018. PMID: 29982706 Free PMC article. Review.
Cited by
-
Corollary discharge enables proprioception from lateral line sensory feedback.PLoS Biol. 2021 Oct 11;19(10):e3001420. doi: 10.1371/journal.pbio.3001420. eCollection 2021 Oct. PLoS Biol. 2021. PMID: 34634044 Free PMC article.
-
Tricaine, eugenol and etomidate for repetitive procedural anesthesia in adult zebrafish, Danio rerio: effect on stress and behavior.Front Vet Sci. 2025 May 14;12:1562425. doi: 10.3389/fvets.2025.1562425. eCollection 2025. Front Vet Sci. 2025. PMID: 40438405 Free PMC article.
-
Water Waves to Sound Waves: Using Zebrafish to Explore Hair Cell Biology.J Assoc Res Otolaryngol. 2019 Feb;20(1):1-19. doi: 10.1007/s10162-018-00711-1. Epub 2019 Jan 11. J Assoc Res Otolaryngol. 2019. PMID: 30635804 Free PMC article. Review.
-
Sensory deficit screen identifies nsf mutation that differentially affects SNARE recycling and quality control.Cell Rep. 2023 Apr 25;42(4):112345. doi: 10.1016/j.celrep.2023.112345. Epub 2023 Apr 5. Cell Rep. 2023. PMID: 37027300 Free PMC article.
-
Sensory adaptation at ribbon synapses in the zebrafish lateral line.J Physiol. 2021 Aug;599(15):3677-3696. doi: 10.1113/JP281646. Epub 2021 Jul 9. J Physiol. 2021. PMID: 34047358 Free PMC article.
References
-
- Assad JA, Shepherd GM & Corey DP (1991). Tip‐link integrity and mechanical transduction in vertebrate hair cells. Neuron 7, 985–994. - PubMed
-
- Barbosa JS, Sanchez‐Gonzalez R, Di Giaimo R, Baumgart EV, Theis FJ, Götz M & Ninkovic J (2015). Live imaging of adult neural stem cell behaviour in the intact and injured zebrafish brain. Science 348, 798–793. - PubMed
-
- Bleckmann H & Zelick R (2009). Lateral line system of fish. Integr Zool 4, 13–25. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

