Weight change in the management of youth-onset type 2 diabetes: the TODAY clinical trial experience
- PMID: 27161901
- PMCID: PMC5209292
- DOI: 10.1111/ijpo.12148
Weight change in the management of youth-onset type 2 diabetes: the TODAY clinical trial experience
Abstract
Background: The Treatment Options for type 2 Diabetes in Adolescents and Youth (TODAY) clinical trial documented that metformin plus rosiglitazone, but not metformin plus lifestyle intervention, provided superior durability of glycemic control relative to metformin monotherapy.
Objectives: We examined weight changes among TODAY participants that completed at least 6 months of treatment, evaluated predictors of lifestyle outcome, and examined whether weight changes were related to cardiometabolic outcomes across treatment arms.
Methods: The 595 youth with type 2 diabetes, (85.1% of randomized participants aged 11-17 years) completed assessments of weight-related and cardiometabolic measures at months 0, 6, 12 and 24. Repeated measures models were used to investigate associations over time.
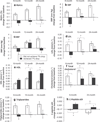
Results: Lifestyle intervention did not enhance outcome relative to metformin alone and no predictors of response to lifestyle treatment were identified. However, changes in percent overweight across treatment arms were associated with changes in multiple cardiometabolic risk factors, and decreases of ≥ 7% in overweight were associated with significant benefits over 24 months.
Conclusions: Although adjunctive intensive lifestyle intervention did not improve weight-related outcomes, weight changes in the full TODAY sample were associated with small, but significant improvements in cardiometabolic status, highlighting the importance of optimizing weight management in youth with T2DM.
Trial registration: ClinicalTrials.gov NCT00081328.
Keywords: behavioral weight control; cardiometabolic risk; lifestyle; type 2 diabetes.
© 2016 World Obesity Federation.
Conflict of interest statement
The TODAY Study Group thanks the following companies for donations in support of the study’s efforts: Becton, Dickinson and Company, Bristol-Myers Squibb, Eli Lilly and Company, GlaxoSmithKline, LifeScan, Inc., Pfizer, and Sanofi-Aventis. MDM is a member of the Scientific Advisory Board of Weight Watchers International, Inc. DEW is a consultant for Shire Pharmaceuticals. PZ is a consultant for Daiichi-Sankyo, Astra-Zeneca, Merck, Takeda, and Eli Lilly. NWA is a consultant for Daiichi-Sankyo. LE, BL, KH, CEIL and DJB have nothing to disclose.
Figures
References
-
- Study Research Group TODAY. Zeitler P, Epstein L, Grey M, et al. Treatment options for type 2 diabetes in adolescents and youth: a study of the comparative efficacy of metformin alone or in combination with rosiglitazone or lifestyle intervention in adolescents with type 2 diabetes. Pediatr Diabetes. 2007;8:74–87. - PMC - PubMed
-
- Kuczmarski RJ, Ogden CL, Guo SS, et al. CDC Growth Charts for the United States: methods and development. Vital Health Stat 11. 2000;246:1–190. - PubMed
-
- Flegal KM, Wei R, Ogden CL, et al. Characterizing extreme values of body mass index-for-age by using the 2000 centers for disease control and prevention growth charts. Am J Clin Nutr. 2009;90:1314–1320. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
- U01 DK061254/DK/NIDDK NIH HHS/United States
- UL1 RR024139/RR/NCRR NIH HHS/United States
- UL1 RR024989/RR/NCRR NIH HHS/United States
- UL1 RR024153/RR/NCRR NIH HHS/United States
- UL1 RR025780/RR/NCRR NIH HHS/United States
- M01 RR000069/RR/NCRR NIH HHS/United States
- M01 RR000036/RR/NCRR NIH HHS/United States
- U01 DK061242/DK/NIDDK NIH HHS/United States
- UL1 RR024992/RR/NCRR NIH HHS/United States
- U01 DK061212/DK/NIDDK NIH HHS/United States
- M01 RR001066/RR/NCRR NIH HHS/United States
- M01 RR014467/RR/NCRR NIH HHS/United States
- U01 DK061230/DK/NIDDK NIH HHS/United States
- T32 AI007172/AI/NIAID NIH HHS/United States
- UL1 TR000448/TR/NCATS NIH HHS/United States
- M01 RR000043/RR/NCRR NIH HHS/United States
- UL1 RR025758/RR/NCRR NIH HHS/United States
- M01 RR000084/RR/NCRR NIH HHS/United States
- UL1 TR002345/TR/NCATS NIH HHS/United States
- M01 RR000125/RR/NCRR NIH HHS/United States
- U01 DK061239/DK/NIDDK NIH HHS/United States
- UL1 RR024134/RR/NCRR NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

