Cyclooxygenase-2 inhibition reduces stress-induced affective pathology
- PMID: 27162170
- PMCID: PMC4862754
- DOI: 10.7554/eLife.14137
Cyclooxygenase-2 inhibition reduces stress-induced affective pathology
Abstract
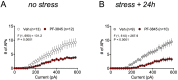
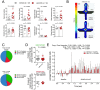
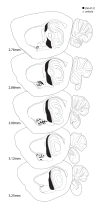
Mood and anxiety disorders are the most prevalent psychiatric conditions and are exacerbated by stress. Recent studies have suggested cyclooxygenase-2 (COX-2) inhibition could represent a novel treatment approach or augmentation strategy for affective disorders including anxiety disorders and major depression. We show that traditional COX-2 inhibitors and a newly developed substrate-selective COX-2 inhibitor (SSCI) reduce a variety of stress-induced behavioral pathologies in mice. We found that these behavioral effects were associated with a dampening of neuronal excitability in the basolateral amygdala (BLA) ex vivo and in vivo, and were mediated by small-conductance calcium-activated potassium (SK) channel and CB1 cannabinoid receptor activation. Taken together, these data provide further support for the potential utility of SSCIs, as well as traditional COX-2 inhibitors, as novel treatment approaches for stress-related psychiatric disorders.
Keywords: COX-2; PTSD; amygdala; anxiety; cannabinoid; mouse; neuroscience; stress.
Conflict of interest statement
The other authors declare that no competing interests exist.
LJM: LJM is a co-inventor on a patent #US20150183737 entitled "Composition and method of Substrate-Selective COX-2 inhibition". LJM has a collaborative research contract with Lundbeck Pharmaceuticals.
SP: SP is a co-inventor on a patent #US20150183737 entitled "Composition and method of Substrate-Selective COX-2 inhibition". SP has a collaborative research contract with Lundbeck Pharmaceuticals.
Figures

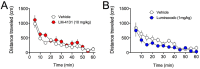

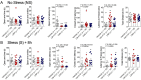
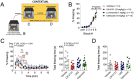
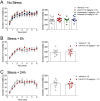



References
-
- Akhondzadeh S, Jafari S, Raisi F, Nasehi AA, Ghoreishi A, Salehi B, Mohebbi-Rasa S, Raznahan M, Kamalipour A. Clinical trial of adjunctive celecoxib treatment in patients with major depression: A double blind and placebo controlled trial. Depression and Anxiety. 2009;26:607–611. doi: 10.1002/da.20589. - DOI - PubMed
-
- Akhondzadeh S, Jafari S. A double-blind, placebo-controlled trial of celecoxib adjunctive treatment to fluoxetine in major depression. British Journal of Clinical Pharmacology. 2010;70:291–292.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

