Blast shockwaves propagate Ca(2+) activity via purinergic astrocyte networks in human central nervous system cells
- PMID: 27162174
- PMCID: PMC4861979
- DOI: 10.1038/srep25713
Blast shockwaves propagate Ca(2+) activity via purinergic astrocyte networks in human central nervous system cells
Abstract
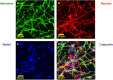
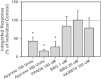
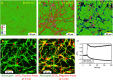
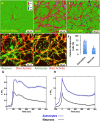
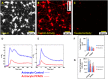
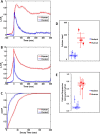
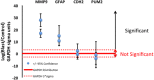
In a recent study of the pathophysiology of mild, blast-induced traumatic brain injury (bTBI) the exposure of dissociated, central nervous system (CNS) cells to simulated blast resulted in propagating waves of elevated intracellular Ca(2+). Here we show, in dissociated human CNS cultures, that these calcium waves primarily propagate through astrocyte-dependent, purinergic signaling pathways that are blocked by P2 antagonists. Human, compared to rat, astrocytes had an increased calcium response and prolonged calcium wave propagation kinetics, suggesting that in our model system rat CNS cells are less responsive to simulated blast. Furthermore, in response to simulated blast, human CNS cells have increased expressions of a reactive astrocyte marker, glial fibrillary acidic protein (GFAP) and a protease, matrix metallopeptidase 9 (MMP-9). The conjoint increased expression of GFAP and MMP-9 and a purinergic ATP (P2) receptor antagonist reduction in calcium response identifies both potential mechanisms for sustained changes in brain function following primary bTBI and therapeutic strategies targeting abnormal astrocyte activity.
Figures

References
-
- Scott S. G., Belanger H. G., Vanderploeg R. D., Massengale J. & Scholten J. Mechanism-of-injury approach to evaluating patients with blast-related polytrauma. J. Am. Osteopath. Assoc. 106, 265–270 (2006). - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

