Prevalent presence of periodic actin-spectrin-based membrane skeleton in a broad range of neuronal cell types and animal species
- PMID: 27162329
- PMCID: PMC4889411
- DOI: 10.1073/pnas.1605707113
Prevalent presence of periodic actin-spectrin-based membrane skeleton in a broad range of neuronal cell types and animal species
Abstract
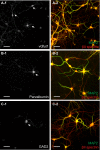
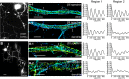
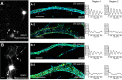
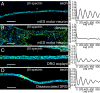
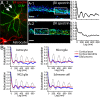
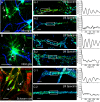
Actin, spectrin, and associated molecules form a periodic, submembrane cytoskeleton in the axons of neurons. For a better understanding of this membrane-associated periodic skeleton (MPS), it is important to address how prevalent this structure is in different neuronal types, different subcellular compartments, and across different animal species. Here, we investigated the organization of spectrin in a variety of neuronal- and glial-cell types. We observed the presence of MPS in all of the tested neuronal types cultured from mouse central and peripheral nervous systems, including excitatory and inhibitory neurons from several brain regions, as well as sensory and motor neurons. Quantitative analyses show that MPS is preferentially formed in axons in all neuronal types tested here: Spectrin shows a long-range, periodic distribution throughout all axons but appears periodic only in a small fraction of dendrites, typically in the form of isolated patches in subregions of these dendrites. As in dendrites, we also observed patches of periodic spectrin structures in a small fraction of glial-cell processes in four types of glial cells cultured from rodent tissues. Interestingly, despite its strong presence in the axonal shaft, MPS is disrupted in most presynaptic boutons but is present in an appreciable fraction of dendritic spine necks, including some projecting from dendrites where such a periodic structure is not observed in the shaft. Finally, we found that spectrin is capable of adopting a similar periodic organization in neurons of a variety of animal species, including Caenorhabditis elegans, Drosophila, Gallus gallus, Mus musculus, and Homo sapiens.
Keywords: STORM; actin; cytoskeleton; neuron; spectrin.
Conflict of interest statement
The authors declare no conflict of interest.
Figures








References
-
- Luo L. Actin cytoskeleton regulation in neuronal morphogenesis and structural plasticity. Annu Rev Cell Dev Biol. 2002;18:601–635. - PubMed
-
- Cingolani LA, Goda Y. Actin in action: The interplay between the actin cytoskeleton and synaptic efficacy. Nat Rev Neurosci. 2008;9(5):344–356. - PubMed
-
- Stiess M, Bradke F. Neuronal polarization: The cytoskeleton leads the way. Dev Neurobiol. 2011;71(6):430–444. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

