Loss of RPGR glutamylation underlies the pathogenic mechanism of retinal dystrophy caused by TTLL5 mutations
- PMID: 27162334
- PMCID: PMC4889371
- DOI: 10.1073/pnas.1523201113
Loss of RPGR glutamylation underlies the pathogenic mechanism of retinal dystrophy caused by TTLL5 mutations
Abstract
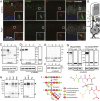
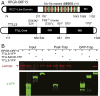
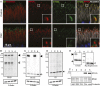
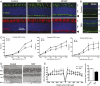
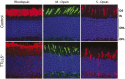
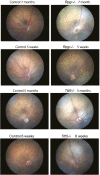
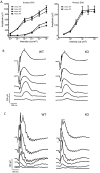
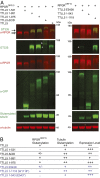
Mutations in the X-linked retinitis pigmentosa GTPase regulator (RPGR) gene are a major cause of retinitis pigmentosa, a blinding retinal disease resulting from photoreceptor degeneration. A photoreceptor specific ORF15 variant of RPGR (RPGR(ORF15)), carrying multiple Glu-Gly tandem repeats and a C-terminal basic domain of unknown function, localizes to the connecting cilium where it is thought to regulate cargo trafficking. Here we show that tubulin tyrosine ligase like-5 (TTLL5) glutamylates RPGR(ORF15) in its Glu-Gly-rich repetitive region containing motifs homologous to the α-tubulin C-terminal tail. The RPGR(ORF15) C-terminal basic domain binds to the noncatalytic cofactor interaction domain unique to TTLL5 among TTLL family glutamylases and targets TTLL5 to glutamylate RPGR. Only TTLL5 and not other TTLL family glutamylases interacts with RPGR(ORF15) when expressed transiently in cells. Consistent with this, a Ttll5 mutant mouse displays a complete loss of RPGR glutamylation without marked changes in tubulin glutamylation levels. The Ttll5 mutant mouse develops slow photoreceptor degeneration with early mislocalization of cone opsins, features resembling those of Rpgr-null mice. Moreover TTLL5 disease mutants that cause human retinal dystrophy show impaired glutamylation of RPGR(ORF15) Thus, RPGR(ORF15) is a novel glutamylation substrate, and this posttranslational modification is critical for its function in photoreceptors. Our study uncovers the pathogenic mechanism whereby absence of RPGR(ORF15) glutamylation leads to retinal pathology in patients with TTLL5 gene mutations and connects these two genes into a common disease pathway.
Keywords: RPGR; cilia; polyglutamylation; retinitis pigmentosa; tubulin tyrosine ligase-like.
Conflict of interest statement
The authors declare no conflict of interest.
Figures




Similar articles
-
Impaired glutamylation of RPGRORF15 underlies the cone-dominated phenotype associated with truncating distal ORF15 variants.Proc Natl Acad Sci U S A. 2022 Dec 6;119(49):e2208707119. doi: 10.1073/pnas.2208707119. Epub 2022 Nov 29. Proc Natl Acad Sci U S A. 2022. PMID: 36445968 Free PMC article.
-
AAV-RPGR Gene Therapy Rescues Opsin Mislocalisation in a Human Retinal Organoid Model of RPGR-Associated X-Linked Retinitis Pigmentosa.Int J Mol Sci. 2024 Feb 2;25(3):1839. doi: 10.3390/ijms25031839. Int J Mol Sci. 2024. PMID: 38339118 Free PMC article.
-
A long-term efficacy study of gene replacement therapy for RPGR-associated retinal degeneration.Hum Mol Genet. 2015 Jul 15;24(14):3956-70. doi: 10.1093/hmg/ddv134. Epub 2015 Apr 15. Hum Mol Genet. 2015. PMID: 25877300 Free PMC article.
-
More Than Meets the Eye: Current Understanding of RPGR Function.Adv Exp Med Biol. 2018;1074:521-538. doi: 10.1007/978-3-319-75402-4_64. Adv Exp Med Biol. 2018. PMID: 29721984 Review.
-
Mutations of RPGR in X-linked retinitis pigmentosa (RP3).Hum Mutat. 2002 May;19(5):486-500. doi: 10.1002/humu.10057. Hum Mutat. 2002. PMID: 11968081 Review.
Cited by
-
Toxicology and Pharmacology of an AAV Vector Expressing Codon-Optimized RPGR in RPGR-Deficient Rd9 Mice.Hum Gene Ther Clin Dev. 2018 Dec;29(4):188-197. doi: 10.1089/humc.2018.168. Hum Gene Ther Clin Dev. 2018. PMID: 30280954 Free PMC article.
-
Retrospective Natural History Study of RPGR-Related Cone- and Cone-Rod Dystrophies While Expanding the Mutation Spectrum of the Disease.Int J Mol Sci. 2022 Jun 28;23(13):7189. doi: 10.3390/ijms23137189. Int J Mol Sci. 2022. PMID: 35806195 Free PMC article.
-
Analysis of RP2 and RPGR Mutations in Five X-Linked Chinese Families with Retinitis Pigmentosa.Sci Rep. 2017 Mar 15;7:44465. doi: 10.1038/srep44465. Sci Rep. 2017. PMID: 28294154 Free PMC article.
-
Antonina Roll-Mecak: Decoding the secrets of tubulin complexity.J Cell Biol. 2017 May 1;216(5):1208-1209. doi: 10.1083/jcb.201703011. Epub 2017 Apr 13. J Cell Biol. 2017. PMID: 28408403 Free PMC article.
-
Glutamylation Regulates Transport, Specializes Function, and Sculpts the Structure of Cilia.Curr Biol. 2017 Nov 20;27(22):3430-3441.e6. doi: 10.1016/j.cub.2017.09.066. Epub 2017 Nov 9. Curr Biol. 2017. PMID: 29129530 Free PMC article.
References
-
- Berson EL. Retinitis pigmentosa. The Friedenwald Lecture. Invest Ophthalmol Vis Sci. 1993;34(5):1659–1676. - PubMed
-
- Bader I, et al. X-linked retinitis pigmentosa: RPGR mutations in most families with definite X linkage and clustering of mutations in a short sequence stretch of exon ORF15. Invest Ophthalmol Vis Sci. 2003;44(4):1458–1463. - PubMed
-
- Pelletier V, et al. Comprehensive survey of mutations in RP2 and RPGR in patients affected with distinct retinal dystrophies: Genotype-phenotype correlations and impact on genetic counseling. Hum Mutat. 2007;28(1):81–91. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

