Enhancement of β-catenin activity by BIG1 plus BIG2 via Arf activation and cAMP signals
- PMID: 27162341
- PMCID: PMC4889387
- DOI: 10.1073/pnas.1601918113
Enhancement of β-catenin activity by BIG1 plus BIG2 via Arf activation and cAMP signals
Abstract
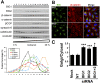
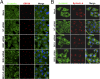
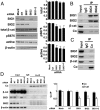
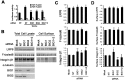
Multifunctional β-catenin, with critical roles in both cell-cell adhesion and Wnt-signaling pathways, was among HeLa cell proteins coimmunoprecipitated by antibodies against brefeldin A-inhibited guanine nucleotide-exchange factors 1 and 2 (BIG1 or BIG2) that activate ADP-ribosylation factors (Arfs) by accelerating the replacement of bound GDP with GTP. BIG proteins also contain A-kinase anchoring protein (AKAP) sequences that can act as scaffolds for multimolecular assemblies that facilitate and limit cAMP signaling temporally and spatially. Direct interaction of BIG1 N-terminal sequence with β-catenin was confirmed using yeast two-hybrid assays and in vitro synthesized proteins. Depletion of BIG1 and/or BIG2 or overexpression of guanine nucleotide-exchange factor inactive mutant, but not wild-type, proteins interfered with β-catenin trafficking, leading to accumulation at perinuclear Golgi structures. Both phospholipase D activity and vesicular trafficking were required for effects of BIG1 and BIG2 on β-catenin activation. Levels of PKA-phosphorylated β-catenin S675 and β-catenin association with PKA, BIG1, and BIG2 were also diminished after BIG1/BIG2 depletion. Inferring a requirement for BIG1 and/or BIG2 AKAP sequence in PKA modification of β-catenin and its effect on transcription activation, we confirmed dependence of S675 phosphorylation and transcription coactivator function on BIG2 AKAP-C sequence.
Keywords: ADP-ribosylation factor; AKAP; cell migration; phospholipase D.
Conflict of interest statement
The authors declare no conflict of interest.
Figures










Similar articles
-
Regulation of brefeldin A-inhibited guanine nucleotide-exchange protein 1 (BIG1) and BIG2 activity via PKA and protein phosphatase 1gamma.Proc Natl Acad Sci U S A. 2007 Feb 27;104(9):3201-6. doi: 10.1073/pnas.0611696104. Epub 2007 Feb 21. Proc Natl Acad Sci U S A. 2007. PMID: 17360629 Free PMC article.
-
Interaction of phosphodiesterase 3A with brefeldin A-inhibited guanine nucleotide-exchange proteins BIG1 and BIG2 and effect on ARF1 activity.Proc Natl Acad Sci U S A. 2009 Apr 14;106(15):6158-63. doi: 10.1073/pnas.0901558106. Epub 2009 Mar 30. Proc Natl Acad Sci U S A. 2009. PMID: 19332778 Free PMC article.
-
BIG1 and BIG2, brefeldin A-inhibited guanine nucleotide-exchange factors for ADP-ribosylation factors.Methods Enzymol. 2005;404:174-84. doi: 10.1016/S0076-6879(05)04017-6. Methods Enzymol. 2005. PMID: 16413268
-
Regulating the regulators: role of phosphorylation in modulating the function of the GBF1/BIG family of Sec7 ARF-GEFs.FEBS Lett. 2020 Jul;594(14):2213-2226. doi: 10.1002/1873-3468.13798. Epub 2020 May 14. FEBS Lett. 2020. PMID: 32333796 Review.
-
Allosteric regulation of Arf GTPases and their GEFs at the membrane interface.Small GTPases. 2016 Oct;7(4):283-296. doi: 10.1080/21541248.2016.1215778. Epub 2016 Jul 22. Small GTPases. 2016. PMID: 27449855 Free PMC article. Review.
Cited by
-
AKAP9 Upregulation Predicts Unfavorable Prognosis in Pediatric Acute Myeloid Leukemia and Promotes Stemness Properties via the Wnt/β-Catenin Pathway.Cancer Manag Res. 2022 Jan 10;14:157-167. doi: 10.2147/CMAR.S343033. eCollection 2022. Cancer Manag Res. 2022. PMID: 35046723 Free PMC article.
-
Walking the tight wire between cell adhesion and WNT signalling: a balancing act for β-catenin.Open Biol. 2020 Dec;10(12):200267. doi: 10.1098/rsob.200267. Epub 2020 Dec 9. Open Biol. 2020. PMID: 33292105 Free PMC article. Review.
-
miR-215 suppresses papillary thyroid cancer proliferation, migration, and invasion through the AKT/GSK-3β/Snail signaling by targeting ARFGEF1.Cell Death Dis. 2019 Feb 27;10(3):195. doi: 10.1038/s41419-019-1444-1. Cell Death Dis. 2019. PMID: 30814512 Free PMC article.
-
Unraveling shared susceptibility loci and Mendelian genetic associations linking educational attainment with multiple neuropsychiatric disorders.Front Psychiatry. 2024 Jan 4;14:1303430. doi: 10.3389/fpsyt.2023.1303430. eCollection 2023. Front Psychiatry. 2024. PMID: 38250258 Free PMC article.
-
miR-139/PDE2A-Notch1 feedback circuit represses stemness of gliomas by inhibiting Wnt/β-catenin signaling.Int J Biol Sci. 2021 Aug 12;17(13):3508-3521. doi: 10.7150/ijbs.62858. eCollection 2021. Int J Biol Sci. 2021. PMID: 34512162 Free PMC article.
References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

