Impaired NK-mediated regulation of T-cell activity in multiple sclerosis is reconstituted by IL-2 receptor modulation
- PMID: 27162345
- PMCID: PMC4889377
- DOI: 10.1073/pnas.1524924113
Impaired NK-mediated regulation of T-cell activity in multiple sclerosis is reconstituted by IL-2 receptor modulation
Abstract
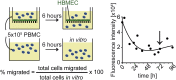
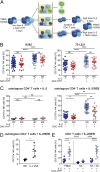
Multiple sclerosis (MS) is a chronic inflammatory autoimmune disease of the central nervous system (CNS) resulting from a breakdown in peripheral immune tolerance. Although a beneficial role of natural killer (NK)-cell immune-regulatory function has been proposed, it still needs to be elucidated whether NK cells are functionally impaired as part of the disease. We observed NK cells in active MS lesions in close proximity to T cells. In accordance with a higher migratory capacity across the blood-brain barrier, CD56(bright) NK cells represent the major intrathecal NK-cell subset in both MS patients and healthy individuals. Investigating the peripheral blood and cerebrospinal fluid of MS patients treated with natalizumab revealed that transmigration of this subset depends on the α4β1 integrin very late antigen (VLA)-4. Although no MS-related changes in the migratory capacity of NK cells were observed, NK cells derived from patients with MS exhibit a reduced cytolytic activity in response to antigen-activated CD4(+) T cells. Defective NK-mediated immune regulation in MS is mainly attributable to a CD4(+) T-cell evasion caused by an impaired DNAX accessory molecule (DNAM)-1/CD155 interaction. Both the expression of the activating NK-cell receptor DNAM-1, a genetic alteration consistently found in MS-association studies, and up-regulation of the receptor's ligand CD155 on CD4(+) T cells are reduced in MS. Therapeutic immune modulation of IL-2 receptor restores impaired immune regulation in MS by increasing the proportion of CD155-expressing CD4(+) T cells and the cytolytic activity of NK cells.
Keywords: DNAM-1; IL-2 receptor; NK cells; daclizumab; multiple sclerosis.
Conflict of interest statement
Conflict of interest statement: A.S.-M., A.R., A.P.-F., T.S.-H., S.H., K.H., M.K., M.H., K.D., R.H., and T.Z. have no financial disclosures. C.C.G. received speaker honoraria and travel expenses for attending meetings from Bayer Health Care, Genzyme, and Novartis Pharma GmbH. T.K. received honoraria for lectures from Novartis, Biogen Idec, Teva, Bayer Health Care, EXCEMED, and Sanofi Aventis, and served as a consultant for Genzyme. N.S. received honoraria for advisory boards and travel expenses from Biogen Idec. L.K. received compensation for serving on scientific advisory boards for Genzyme. L.K. received speaker honoraria and travel support from Novartis, Merck Serono, and CSL Behring. L.K. receives research support from Novartis. S.G.M. received honoraria for lecturing, travel expenses for attending meetings, and financial research support from Almirall, Bayer Health Care, Biogen, Genzyme, Merck Serono, Novartis, Novo Nordisk, Roche, Sanofi-Aventis, and Teva. H.W. received compensation for serving on Scientific Advisory Boards/Steering Committees for Bayer Healthcare, Biogen Idec, Genzyme, Merck Serono, Novartis, and Sanofi Aventis. H.W. also received speaker honoraria and travel support from Bayer Vital GmbH, Bayer Schering AG, Biogen Idec, CSL Behring, Fresenius Medical Care, Genzyme, Glaxo Smith Kline, GW Pharmaceuticals, Lundbeck, Merck Serono, Omniamed, Novartis, and Sanofi-Aventis. H.W. received compensation as a consultant from Biogen Idec, Merck Serono, Novartis, and Sanofi-Aventis. H.W. received research support from Bayer Vital, Biogen Idec, Genzyme Merck Serono, Novartis, Sanofi-Aventis Germany, and Sanofi US.
Figures










Comment in
-
Reply to Liu et al.: Haplotype matters: CD226 polymorphism as a potential trigger for impaired immune regulation in multiple sclerosis.Proc Natl Acad Sci U S A. 2017 Feb 7;114(6):E908-E909. doi: 10.1073/pnas.1619059114. Epub 2017 Jan 30. Proc Natl Acad Sci U S A. 2017. PMID: 28137888 Free PMC article. No abstract available.
-
Genetic variant rs763361 regulates multiple sclerosis CD226 gene expression.Proc Natl Acad Sci U S A. 2017 Feb 7;114(6):E906-E907. doi: 10.1073/pnas.1618520114. Epub 2017 Jan 30. Proc Natl Acad Sci U S A. 2017. PMID: 28137889 Free PMC article. No abstract available.
References
-
- Dendrou CA, Fugger L, Friese MA. Immunopathology of multiple sclerosis. Nat Rev Immunol. 2015;15(9):545–558. - PubMed
-
- Wiendl H, Gross CC. Modulation of IL-2Rα with daclizumab for treatment of multiple sclerosis. Nat Rev Neurol. 2013;9(7):394–404. - PubMed
-
- Cerboni C, et al. Antigen-activated human T lymphocytes express cell-surface NKG2D ligands via an ATM/ATR-dependent mechanism and become susceptible to autologous NK-cell lysis. Blood. 2007;110(2):606–615. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

