Architecture of retinal projections to the central circadian pacemaker
- PMID: 27162356
- PMCID: PMC4889372
- DOI: 10.1073/pnas.1523629113
Architecture of retinal projections to the central circadian pacemaker
Abstract
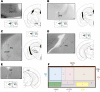
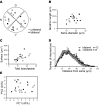
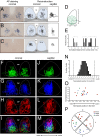
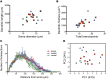
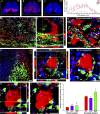
The suprachiasmatic nucleus (SCN) receives direct retinal input from the intrinsically photosensitive retinal ganglion cells (ipRGCs) for circadian photoentrainment. Interestingly, the SCN is the only brain region that receives equal inputs from the left and right eyes. Despite morphological assessments showing that axonal fibers originating from ipRGCs cover the entire SCN, physiological evidence suggests that only vasoactive intestinal polypeptide (VIP)/gastrin-releasing peptide (GRP) cells located ventrally in the SCN receive retinal input. It is still unclear, therefore, which subpopulation of SCN neurons receives synaptic input from the retina and how the SCN receives equal inputs from both eyes. Here, using single ipRGC axonal tracing and a confocal microscopic analysis in mice, we show that ipRGCs have elaborate innervation patterns throughout the entire SCN. Unlike conventional retinal ganglion cells (RGCs) that innervate visual targets either ipsilaterally or contralaterally, a single ipRGC can bilaterally innervate the SCN. ipRGCs form synaptic contacts with major peptidergic cells of the SCN, including VIP, GRP, and arginine vasopressin (AVP) neurons, with each ipRGC innervating specific subdomains of the SCN. Furthermore, a single SCN-projecting ipRGC can send collateral inputs to many other brain regions. However, the size and complexity of the axonal arborizations in non-SCN regions are less elaborate than those in the SCN. Our results provide a better understanding of how retinal neurons connect to the central circadian pacemaker to synchronize endogenous circadian clocks with the solar day.
Keywords: circadian; ipRGCs; melanopsin; non-image–forming functions; suprachiasmatic nucleus.
Conflict of interest statement
The authors declare no conflict of interest.
Figures









Similar articles
-
Two types of melanopsin retinal ganglion cell differentially innervate the hypothalamic suprachiasmatic nucleus and the olivary pretectal nucleus.Eur J Neurosci. 2008 Apr;27(7):1763-70. doi: 10.1111/j.1460-9568.2008.06149.x. Epub 2008 Mar 25. Eur J Neurosci. 2008. PMID: 18371076
-
Degeneration of ipRGCs in Mouse Models of Huntington's Disease Disrupts Non-Image-Forming Behaviors Before Motor Impairment.J Neurosci. 2019 Feb 20;39(8):1505-1524. doi: 10.1523/JNEUROSCI.0571-18.2018. Epub 2018 Dec 26. J Neurosci. 2019. PMID: 30587542 Free PMC article.
-
Intrinsically Photosensitive Retinal Ganglion Cells (ipRGCs) Are Necessary for Light Entrainment of Peripheral Clocks.PLoS One. 2016 Dec 16;11(12):e0168651. doi: 10.1371/journal.pone.0168651. eCollection 2016. PLoS One. 2016. PMID: 27992553 Free PMC article.
-
Melanopsin--shedding light on the elusive circadian photopigment.Chronobiol Int. 2004 Mar;21(2):189-204. doi: 10.1081/cbi-120037816. Chronobiol Int. 2004. PMID: 15332341 Free PMC article. Review.
-
Genetic advances in ophthalmology: the role of melanopsin-expressing, intrinsically photosensitive retinal ganglion cells in the circadian organization of the visual system.Semin Ophthalmol. 2013 Sep-Nov;28(5-6):406-21. doi: 10.3109/08820538.2013.825294. Epub 2013 Sep 6. Semin Ophthalmol. 2013. PMID: 24010846 Review.
Cited by
-
Synaptic Specializations of Melanopsin-Retinal Ganglion Cells in Multiple Brain Regions Revealed by Genetic Label for Light and Electron Microscopy.Cell Rep. 2019 Oct 15;29(3):628-644.e6. doi: 10.1016/j.celrep.2019.09.006. Cell Rep. 2019. PMID: 31618632 Free PMC article.
-
Biophysical Variation within the M1 Type of Ganglion Cell Photoreceptor.Cell Rep. 2017 Oct 24;21(4):1048-1062. doi: 10.1016/j.celrep.2017.09.095. Cell Rep. 2017. PMID: 29069587 Free PMC article.
-
Melatonin promotes sleep by activating the BK channel in C. elegans.Proc Natl Acad Sci U S A. 2020 Oct 6;117(40):25128-25137. doi: 10.1073/pnas.2010928117. Epub 2020 Sep 21. Proc Natl Acad Sci U S A. 2020. PMID: 32958651 Free PMC article.
-
A non-canonical retina-ipRGCs-SCN-PVT visual pathway for mediating contagious itch behavior.Cell Rep. 2022 Oct 4;41(1):111444. doi: 10.1016/j.celrep.2022.111444. Cell Rep. 2022. PMID: 36198265 Free PMC article.
-
From Fast Oscillations to Circadian Rhythms: Coupling at Multiscale Frequency Bands in the Rodent Subcortical Visual System.Front Physiol. 2021 Nov 26;12:738229. doi: 10.3389/fphys.2021.738229. eCollection 2021. Front Physiol. 2021. PMID: 34899375 Free PMC article.
References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

