Hedgehog signaling enables nutrition-responsive inhibition of an alternative morph in a polyphenic beetle
- PMID: 27162357
- PMCID: PMC4889385
- DOI: 10.1073/pnas.1601505113
Hedgehog signaling enables nutrition-responsive inhibition of an alternative morph in a polyphenic beetle
Abstract
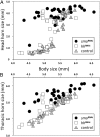
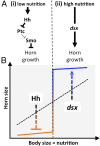
The recruitment of modular developmental genetic components into new developmental contexts has been proposed as a central mechanism enabling the origin of novel traits and trait functions without necessitating the origin of novel pathways. Here, we investigate the function of the hedgehog (Hh) signaling pathway, a highly conserved pathway best understood for its role in patterning anterior/posterior (A/P) polarity of diverse traits, in the developmental evolution of beetle horns, an evolutionary novelty, and horn polyphenisms, a highly derived form of environment-responsive trait induction. We show that interactions among pathway members are conserved during development of Onthophagus horned beetles and have retained the ability to regulate A/P polarity in traditional appendages, such as legs. At the same time, the Hh signaling pathway has acquired a novel and highly unusual role in the nutrition-dependent regulation of horn polyphenisms by actively suppressing horn formation in low-nutrition males. Down-regulation of Hh signaling lifts this inhibition and returns a highly derived sigmoid horn body size allometry to its presumed ancestral, linear state. Our results suggest that recruitment of the Hh signaling pathway may have been a key step in the evolution of trait thresholds, such as those involved in horn polyphenisms and the corresponding origin of alternative phenotypes and complex allometries.
Keywords: allometry; co-option; developmental plasticity; modularity; threshold trait.
Conflict of interest statement
The authors declare no conflict of interest.
Figures




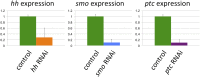
Similar articles
-
Insulin signalling's role in mediating tissue-specific nutritional plasticity and robustness in the horn-polyphenic beetle Onthophagus taurus.Proc Biol Sci. 2018 Dec 19;285(1893):20181631. doi: 10.1098/rspb.2018.1631. Proc Biol Sci. 2018. PMID: 30963895 Free PMC article.
-
Serotonin signaling suppresses the nutrition-responsive induction of an alternate male morph in horn polyphenic beetles.J Exp Zool A Ecol Integr Physiol. 2020 Nov;333(9):660-669. doi: 10.1002/jez.2413. Epub 2020 Sep 22. J Exp Zool A Ecol Integr Physiol. 2020. PMID: 32959988
-
Developmental regulation and evolution of scaling: novel insights through the study of Onthophagus beetles.Curr Opin Insect Sci. 2017 Feb;19:52-60. doi: 10.1016/j.cois.2016.11.004. Epub 2016 Dec 5. Curr Opin Insect Sci. 2017. PMID: 28521943 Review.
-
Notch signaling patterns head horn shape in the bull-headed dung beetle Onthophagus taurus.Dev Genes Evol. 2020 May;230(3):213-225. doi: 10.1007/s00427-020-00645-w. Epub 2020 Jan 20. Dev Genes Evol. 2020. PMID: 31960122
-
Insulin signaling and limb-patterning: candidate pathways for the origin and evolutionary diversification of beetle 'horns'.Heredity (Edinb). 2006 Sep;97(3):179-91. doi: 10.1038/sj.hdy.6800868. Epub 2006 Jul 19. Heredity (Edinb). 2006. PMID: 16850039 Review.
Cited by
-
Genome evolution and divergence in cis-regulatory architecture is associated with condition-responsive development in horned dung beetles.PLoS Genet. 2024 Mar 5;20(3):e1011165. doi: 10.1371/journal.pgen.1011165. eCollection 2024 Mar. PLoS Genet. 2024. PMID: 38442113 Free PMC article.
-
Polyphenism of a Novel Trait Integrated Rapidly Evolving Genes into Ancestrally Plastic Networks.Mol Biol Evol. 2021 Jan 23;38(2):331-343. doi: 10.1093/molbev/msaa235. Mol Biol Evol. 2021. PMID: 32931588 Free PMC article.
-
Nutrition-responsive gene expression and the developmental evolution of insect polyphenism.Nat Ecol Evol. 2020 Jul;4(7):970-978. doi: 10.1038/s41559-020-1202-x. Epub 2020 May 18. Nat Ecol Evol. 2020. PMID: 32424280
-
Hedgehog signaling is necessary and sufficient to mediate craniofacial plasticity in teleosts.Proc Natl Acad Sci U S A. 2020 Aug 11;117(32):19321-19327. doi: 10.1073/pnas.1921856117. Epub 2020 Jul 27. Proc Natl Acad Sci U S A. 2020. PMID: 32719137 Free PMC article.
-
Gene regulatory networks underlying the development and evolution of plasticity in horned beetles.Curr Opin Insect Sci. 2023 Dec;60:101114. doi: 10.1016/j.cois.2023.101114. Epub 2023 Sep 13. Curr Opin Insect Sci. 2023. PMID: 37709168 Free PMC article. Review.
References
-
- Shubin N, Tabin C, Carroll S. Deep homology and the origins of evolutionary novelty. Nature. 2009;457(7231):818–823. - PubMed
-
- Monteiro A. Origin, development, and evolution of butterfly eyespots. Annu Rev Entomol. 2015;60:253–271. - PubMed
-
- Brunetti CR, et al. The generation and diversification of butterfly eyespot color patterns. Curr Biol. 2001;11(20):1578–1585. - PubMed
-
- Pfennig DW, et al. Phenotypic plasticity’s impacts on diversification and speciation. Trends Ecol Evol. 2010;25(8):459–467. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

