Glutathione adducts induced by ischemia and deletion of glutaredoxin-1 stabilize HIF-1α and improve limb revascularization
- PMID: 27162359
- PMCID: PMC4889374
- DOI: 10.1073/pnas.1524198113
Glutathione adducts induced by ischemia and deletion of glutaredoxin-1 stabilize HIF-1α and improve limb revascularization
Abstract
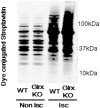
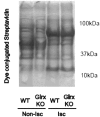
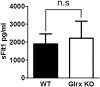
Reactive oxygen species (ROS) are increased in ischemic tissues and necessary for revascularization; however, the mechanism remains unclear. Exposure of cysteine residues to ROS in the presence of glutathione (GSH) generates GSH-protein adducts that are specifically reversed by the cytosolic thioltransferase, glutaredoxin-1 (Glrx). Here, we show that a key angiogenic transcriptional factor hypoxia-inducible factor (HIF)-1α is stabilized by GSH adducts, and the genetic deletion of Glrx improves ischemic revascularization. In mouse muscle C2C12 cells, HIF-1α protein levels are increased by increasing GSH adducts with cell-permeable oxidized GSH (GSSG-ethyl ester) or 2-acetylamino-3-[4-(2-acetylamino-2-carboxyethylsulfanyl thiocarbonylamino) phenylthiocarbamoylsulfanyl] propionic acid (2-AAPA), an inhibitor of glutathione reductase. A biotin switch assay shows that GSSG-ester-induced HIF-1α contains reversibly modified thiols, and MS confirms GSH adducts on Cys(520) (mouse Cys(533)). In addition, an HIF-1α Cys(520) serine mutant is resistant to 2-AAPA-induced HIF-1α stabilization. Furthermore, Glrx overexpression prevents HIF-1α stabilization, whereas Glrx ablation by siRNA increases HIF-1α protein and expression of downstream angiogenic genes. Blood flow recovery after femoral artery ligation is significantly improved in Glrx KO mice, associated with increased levels of GSH-protein adducts, capillary density, vascular endothelial growth factor (VEGF)-A, and HIF-1α in the ischemic muscles. Therefore, Glrx ablation stabilizes HIF-1α by increasing GSH adducts on Cys(520) promoting in vivo HIF-1α stabilization, VEGF-A production, and revascularization in the ischemic muscles.
Keywords: GSH-protein adducts; S-glutathionylation; glutaredoxin-1; hypoxia-inducible factor-1; ischemic limb revascularization.
Conflict of interest statement
The authors declare no conflict of interest.
Figures








Similar articles
-
Redox regulation of ischemic limb neovascularization - What we have learned from animal studies.Redox Biol. 2017 Aug;12:1011-1019. doi: 10.1016/j.redox.2017.04.040. Epub 2017 May 4. Redox Biol. 2017. PMID: 28505880 Free PMC article. Review.
-
Glutaredoxin-1 up-regulation induces soluble vascular endothelial growth factor receptor 1, attenuating post-ischemia limb revascularization.J Biol Chem. 2014 Mar 21;289(12):8633-44. doi: 10.1074/jbc.M113.517219. Epub 2014 Jan 30. J Biol Chem. 2014. PMID: 24482236 Free PMC article.
-
Effect of pioglitazone on expression of hypoxia-inducible factor 1α and vascular endothelial growth factor in ischemic hindlimb of diabetic rats.Eur Rev Med Pharmacol Sci. 2014;18(9):1307-14. Eur Rev Med Pharmacol Sci. 2014. PMID: 24867508
-
Deletion of prolyl hydroxylase domain proteins (PHD1, PHD3) stabilizes hypoxia inducible factor-1 alpha, promotes neovascularization, and improves perfusion in a murine model of hind-limb ischemia.Microvasc Res. 2015 Jan;97:181-8. doi: 10.1016/j.mvr.2014.10.009. Epub 2014 Nov 3. Microvasc Res. 2015. PMID: 25446011
-
Redox Regulation of Ischemic Angiogenesis - Another Aspect of Reactive Oxygen Species.Circ J. 2016 May 25;80(6):1278-84. doi: 10.1253/circj.CJ-16-0317. Epub 2016 May 6. Circ J. 2016. PMID: 27151566 Free PMC article. Review.
Cited by
-
Hypoxia, oxidative stress, and the interplay of HIFs and NRF2 signaling in cancer.Exp Mol Med. 2024 Mar;56(3):501-514. doi: 10.1038/s12276-024-01180-8. Epub 2024 Mar 1. Exp Mol Med. 2024. PMID: 38424190 Free PMC article. Review.
-
Oxidative Stress: Signaling Pathways, Biological Functions, and Disease.MedComm (2020). 2025 Jul 1;6(7):e70268. doi: 10.1002/mco2.70268. eCollection 2025 Jul. MedComm (2020). 2025. PMID: 40599237 Free PMC article. Review.
-
A conserved cysteine-based redox mechanism sustains TFEB/HLH-30 activity under persistent stress.EMBO J. 2021 Feb 1;40(3):e105793. doi: 10.15252/embj.2020105793. Epub 2020 Dec 14. EMBO J. 2021. PMID: 33314217 Free PMC article.
-
Dysregulation of the glutaredoxin/S-glutathionylation redox axis in lung diseases.Am J Physiol Cell Physiol. 2020 Feb 1;318(2):C304-C327. doi: 10.1152/ajpcell.00410.2019. Epub 2019 Nov 6. Am J Physiol Cell Physiol. 2020. PMID: 31693398 Free PMC article.
-
Ischemic perconditioning on mesenteric ischemia/reperfusion injury in rats.Acta Cir Bras. 2021 Nov 8;36(9):e360903. doi: 10.1590/ACB360903. eCollection 2021. Acta Cir Bras. 2021. PMID: 34755763 Free PMC article.
References
-
- Kris-Etherton PM, Lichtenstein AH, Howard BV, Steinberg D, Witztum JL. Nutrition Committee of the American Heart Association Council on Nutrition, Physical Activity, and Metabolism Antioxidant vitamin supplements and cardiovascular disease. Circulation. 2004;110(5):637–641. - PubMed
-
- Tojo T, et al. Role of gp91phox (Nox2)-containing NAD(P)H oxidase in angiogenesis in response to hindlimb ischemia. Circulation. 2005;111(18):2347–2355. - PubMed
-
- Ichihara S, et al. Ablation of the transcription factor Nrf2 promotes ischemia-induced neovascularization by enhancing the inflammatory response. Arterioscler Thromb Vasc Biol. 2010;30(8):1553–1561. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

