Endogenous B-ring oxysterols inhibit the Hedgehog component Smoothened in a manner distinct from cyclopamine or side-chain oxysterols
- PMID: 27162362
- PMCID: PMC4889404
- DOI: 10.1073/pnas.1604984113
Endogenous B-ring oxysterols inhibit the Hedgehog component Smoothened in a manner distinct from cyclopamine or side-chain oxysterols
Abstract
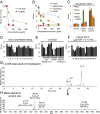
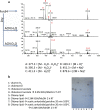
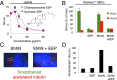
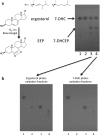
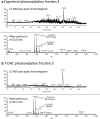
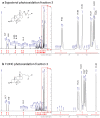
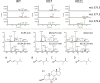
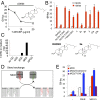
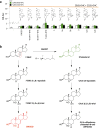
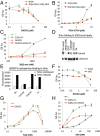
Cellular lipids are speculated to act as key intermediates in Hedgehog signal transduction, but their precise identity and function remain enigmatic. In an effort to identify such lipids, we pursued a Hedgehog pathway inhibitory activity that is particularly abundant in flagellar lipids of Chlamydomonas reinhardtii, resulting in the purification and identification of ergosterol endoperoxide, a B-ring oxysterol. A mammalian analog of ergosterol, 7-dehydrocholesterol (7-DHC), accumulates in Smith-Lemli-Opitz syndrome, a human genetic disease that phenocopies deficient Hedgehog signaling and is caused by genetic loss of 7-DHC reductase. We found that depleting endogenous 7-DHC with methyl-β-cyclodextrin treatment enhances Hedgehog activation by a pathway agonist. Conversely, exogenous addition of 3β,5α-dihydroxycholest-7-en-6-one, a naturally occurring B-ring oxysterol derived from 7-DHC that also accumulates in Smith-Lemli-Opitz syndrome, blocked Hedgehog signaling by inhibiting activation of the essential transduction component Smoothened, through a mechanism distinct from Smoothened modulation by other lipids.
Keywords: DHCEO; Hedgehog signaling; SLOS; Smoothened; cyclodextrin.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


Similar articles
-
An oxysterol biomarker for 7-dehydrocholesterol oxidation in cell/mouse models for Smith-Lemli-Opitz syndrome.J Lipid Res. 2011 Jun;52(6):1222-1233. doi: 10.1194/jlr.M014498. Epub 2011 Mar 14. J Lipid Res. 2011. PMID: 21402677 Free PMC article.
-
7-Dehydrocholesterol-derived oxysterols and retinal degeneration in a rat model of Smith-Lemli-Opitz syndrome.Biochim Biophys Acta. 2012 Jun;1821(6):877-83. doi: 10.1016/j.bbalip.2012.03.001. Epub 2012 Mar 9. Biochim Biophys Acta. 2012. PMID: 22425966 Free PMC article.
-
Lipid biomarkers of oxidative stress in a genetic mouse model of Smith-Lemli-Opitz syndrome.J Inherit Metab Dis. 2013 Jan;36(1):113-22. doi: 10.1007/s10545-012-9504-z. Epub 2012 Jun 21. J Inherit Metab Dis. 2013. PMID: 22718275 Free PMC article.
-
Hedgehog signaling update.Am J Med Genet A. 2010 Aug;152A(8):1875-914. doi: 10.1002/ajmg.a.32909. Am J Med Genet A. 2010. PMID: 20635334 Review.
-
Roughing up Smoothened: chemical modulators of hedgehog signaling.J Biol. 2002 Nov 6;1(2):8. doi: 10.1186/1475-4924-1-8. J Biol. 2002. PMID: 12437769 Free PMC article. Review.
Cited by
-
The Intimate Connection Between Lipids and Hedgehog Signaling.Front Cell Dev Biol. 2022 Jun 9;10:876815. doi: 10.3389/fcell.2022.876815. eCollection 2022. Front Cell Dev Biol. 2022. PMID: 35757007 Free PMC article. Review.
-
Unravelling new pathways of sterol metabolism: lessons learned from in-born errors and cancer.Curr Opin Clin Nutr Metab Care. 2018 Mar;21(2):90-96. doi: 10.1097/MCO.0000000000000442. Curr Opin Clin Nutr Metab Care. 2018. PMID: 29227331 Free PMC article. Review.
-
Smith-Lemli-Opitz syndrome: A pathophysiological manifestation of the Bloch hypothesis.Front Mol Biosci. 2023 Jan 12;10:1120373. doi: 10.3389/fmolb.2023.1120373. eCollection 2023. Front Mol Biosci. 2023. PMID: 36714259 Free PMC article. Review.
-
An update on oxysterol biochemistry: New discoveries in lipidomics.Biochem Biophys Res Commun. 2018 Oct 7;504(3):617-622. doi: 10.1016/j.bbrc.2018.02.019. Epub 2018 Feb 5. Biochem Biophys Res Commun. 2018. PMID: 29421651 Free PMC article. Review.
-
Rapid, direct activity assays for Smoothened reveal Hedgehog pathway regulation by membrane cholesterol and extracellular sodium.Proc Natl Acad Sci U S A. 2017 Dec 26;114(52):E11141-E11150. doi: 10.1073/pnas.1717891115. Epub 2017 Dec 11. Proc Natl Acad Sci U S A. 2017. PMID: 29229834 Free PMC article.
References
-
- Briscoe J, Thérond PP. The mechanisms of Hedgehog signalling and its roles in development and disease. Nat Rev Mol Cell Biol. 2013;14(7):416–429. - PubMed
-
- Chiang C, et al. Cyclopia and defective axial patterning in mice lacking Sonic hedgehog gene function. Nature. 1996;383(6599):407–413. - PubMed
-
- Roessler E, et al. Mutations in the human Sonic hedgehog gene cause holoprosencephaly. Nat Genet. 1996;14(3):357–360. - PubMed
-
- Belloni E, et al. Identification of Sonic hedgehog as a candidate gene responsible for holoprosencephaly. Nat Genet. 1996;14(3):353–356. - PubMed
-
- Taipale J, Cooper MK, Maiti T, Beachy PA. Patched acts catalytically to suppress the activity of Smoothened. Nature. 2002;418(6900):892–897. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

