PML plays both inimical and beneficial roles in HSV-1 replication
- PMID: 27162364
- PMCID: PMC4889406
- DOI: 10.1073/pnas.1605513113
PML plays both inimical and beneficial roles in HSV-1 replication
Abstract
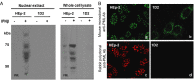
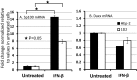
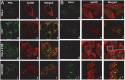
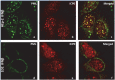
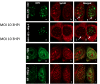
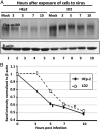
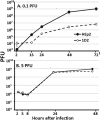
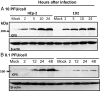
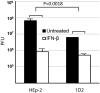
After entry into the nucleus, herpes simplex virus (HSV) DNA is coated with repressive proteins and becomes the site of assembly of nuclear domain 10 (ND10) bodies. These small (0.1-1 μM) nuclear structures contain both constant [e.g., promyelocytic leukemia protein (PML), Sp100, death-domain associated protein (Daxx), and so forth] and variable proteins, depending on the function of the cells or the stress to which they are exposed. The amounts of PML and the number of ND10 structures increase in cells exposed to IFN-β. On initiation of HSV-1 gene expression, ICP0, a viral E3 ligase, degrades both PML and Sp100. The earlier report that IFN-β is significantly more effective in blocking viral replication in murine PML(+/+) cells than in sibling PML(-/-) cells, reproduced here with human cells, suggests that PML acts as an effector of antiviral effects of IFN-β. To define more precisely the function of PML in HSV-1 replication, we constructed a PML(-/-) human cell line. We report that in PML(-/-) cells, Sp100 degradation is delayed, possibly because colocalization and merger of ICP0 with nuclear bodies containing Sp100 and Daxx is ineffective, and that HSV-1 replicates equally well in parental HEp-2 and PML(-/-) cells infected at 5 pfu wild-type virus per cell, but poorly in PML(-/-) cells exposed to 0.1 pfu per cell. Finally, ICP0 accumulation is reduced in PML(-/-) infected at low, but not high, multiplicities of infection. In essence, the very mechanism that serves to degrade an antiviral IFN-β effector is exploited by HSV-1 to establish an efficient replication domain in the nucleus.
Keywords: Daxx; ICP0; ND10; Sp100; interferon.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
Similar articles
-
The SP100 component of ND10 enhances accumulation of PML and suppresses replication and the assembly of HSV replication compartments.Proc Natl Acad Sci U S A. 2017 May 9;114(19):E3823-E3829. doi: 10.1073/pnas.1703395114. Epub 2017 Apr 24. Proc Natl Acad Sci U S A. 2017. PMID: 28439026 Free PMC article.
-
Two overlapping regions within the N-terminal half of the herpes simplex virus 1 E3 ubiquitin ligase ICP0 facilitate the degradation and dissociation of PML and dissociation of Sp100 from ND10.J Virol. 2013 Dec;87(24):13287-96. doi: 10.1128/JVI.02304-13. Epub 2013 Oct 2. J Virol. 2013. PMID: 24089549 Free PMC article.
-
Expression of Human Cytomegalovirus IE1 Leads to Accumulation of Mono-SUMOylated PML That Is Protected from Degradation by Herpes Simplex Virus 1 ICP0.J Virol. 2018 Nov 12;92(23):e01452-18. doi: 10.1128/JVI.01452-18. Print 2018 Dec 1. J Virol. 2018. PMID: 30258013 Free PMC article.
-
The potential link between PML NBs and ICP0 in regulating lytic and latent infection of HSV-1.Protein Cell. 2012 May;3(5):372-82. doi: 10.1007/s13238-012-2021-x. Epub 2012 Apr 28. Protein Cell. 2012. PMID: 22544561 Free PMC article. Review.
-
Emerging Role of PML Nuclear Bodies in Innate Immune Signaling.J Virol. 2016 Jun 10;90(13):5850-5854. doi: 10.1128/JVI.01979-15. Print 2016 Jul 1. J Virol. 2016. PMID: 27053550 Free PMC article. Review.
Cited by
-
KSHV hijacks the antiviral kinase IKKε to initiate lytic replication.PLoS Pathog. 2025 Jan 17;21(1):e1012856. doi: 10.1371/journal.ppat.1012856. eCollection 2025 Jan. PLoS Pathog. 2025. PMID: 39823515 Free PMC article.
-
Importance of Promyelocytic Leukema Protein (PML) for Kaposi's Sarcoma-Associated Herpesvirus Lytic Replication.Front Microbiol. 2018 Oct 8;9:2324. doi: 10.3389/fmicb.2018.02324. eCollection 2018. Front Microbiol. 2018. PMID: 30349510 Free PMC article.
-
The Role of ND10 Nuclear Bodies in Herpesvirus Infection: A Frenemy for the Virus?Viruses. 2021 Feb 3;13(2):239. doi: 10.3390/v13020239. Viruses. 2021. PMID: 33546431 Free PMC article. Review.
-
Sp100 colocalizes with HPV replication foci and restricts the productive stage of the infectious cycle.PLoS Pathog. 2017 Oct 2;13(10):e1006660. doi: 10.1371/journal.ppat.1006660. eCollection 2017 Oct. PLoS Pathog. 2017. PMID: 28968443 Free PMC article.
-
Promyelocytic Leukemia Restricts Enterovirus 71 Replication by Inhibiting Autophagy.Front Immunol. 2018 Jun 5;9:1268. doi: 10.3389/fimmu.2018.01268. eCollection 2018. Front Immunol. 2018. PMID: 29922292 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

