Primer release is the rate-limiting event in lagging-strand synthesis mediated by the T7 replisome
- PMID: 27162371
- PMCID: PMC4889403
- DOI: 10.1073/pnas.1604894113
Primer release is the rate-limiting event in lagging-strand synthesis mediated by the T7 replisome
Abstract
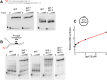
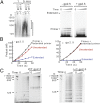
DNA replication occurs semidiscontinuously due to the antiparallel DNA strands and polarity of enzymatic DNA synthesis. Although the leading strand is synthesized continuously, the lagging strand is synthesized in small segments designated Okazaki fragments. Lagging-strand synthesis is a complex event requiring repeated cycles of RNA primer synthesis, transfer to the lagging-strand polymerase, and extension effected by cooperation between DNA primase and the lagging-strand polymerase. We examined events controlling Okazaki fragment initiation using the bacteriophage T7 replication system. Primer utilization by T7 DNA polymerase is slower than primer formation. Slow primer release from DNA primase allows the polymerase to engage the complex and is followed by a slow primer handoff step. The T7 single-stranded DNA binding protein increases primer formation and extension efficiency but promotes limited rounds of primer extension. We present a model describing Okazaki fragment initiation, the regulation of fragment length, and their implications for coordinated leading- and lagging-strand DNA synthesis.
Keywords: DNA primase; Okazaki fragment; primer; replisome.
Conflict of interest statement
The authors declare no conflict of interest.
Figures









Similar articles
-
RNA primer-primase complexes serve as the signal for polymerase recycling and Okazaki fragment initiation in T4 phage DNA replication.Proc Natl Acad Sci U S A. 2017 May 30;114(22):5635-5640. doi: 10.1073/pnas.1620459114. Epub 2017 May 15. Proc Natl Acad Sci U S A. 2017. PMID: 28507156 Free PMC article.
-
Lagging strand synthesis in coordinated DNA synthesis by bacteriophage t7 replication proteins.J Mol Biol. 2002 Feb 8;316(1):19-34. doi: 10.1006/jmbi.2001.5325. J Mol Biol. 2002. PMID: 11829500
-
Binding Affinities among DNA Helicase-Primase, DNA Polymerase, and Replication Intermediates in the Replisome of Bacteriophage T7.J Biol Chem. 2016 Jan 15;291(3):1472-80. doi: 10.1074/jbc.M115.698233. Epub 2015 Nov 30. J Biol Chem. 2016. PMID: 26620561 Free PMC article.
-
Motors, switches, and contacts in the replisome.Annu Rev Biochem. 2009;78:205-43. doi: 10.1146/annurev.biochem.78.072407.103248. Annu Rev Biochem. 2009. PMID: 19298182 Review.
-
Days weaving the lagging strand synthesis of DNA - A personal recollection of the discovery of Okazaki fragments and studies on discontinuous replication mechanism.Proc Jpn Acad Ser B Phys Biol Sci. 2017;93(5):322-338. doi: 10.2183/pjab.93.020. Proc Jpn Acad Ser B Phys Biol Sci. 2017. PMID: 28496054 Free PMC article. Review.
Cited by
-
Modulation of RNA primer formation by Mn(II)-substituted T7 DNA primase.Sci Rep. 2017 Jul 19;7(1):5797. doi: 10.1038/s41598-017-05534-3. Sci Rep. 2017. PMID: 28724886 Free PMC article.
-
Bacillus subtilis DNA polymerases, PolC and DnaE, are required for both leading and lagging strand synthesis in SPP1 origin-dependent DNA replication.Nucleic Acids Res. 2017 Aug 21;45(14):8302-8313. doi: 10.1093/nar/gkx493. Nucleic Acids Res. 2017. PMID: 28575448 Free PMC article.
-
Gp2.5, the multifunctional bacteriophage T7 single-stranded DNA binding protein.Semin Cell Dev Biol. 2019 Feb;86:92-101. doi: 10.1016/j.semcdb.2018.03.018. Epub 2018 Mar 28. Semin Cell Dev Biol. 2019. PMID: 29588157 Free PMC article. Review.
-
RNA primer-primase complexes serve as the signal for polymerase recycling and Okazaki fragment initiation in T4 phage DNA replication.Proc Natl Acad Sci U S A. 2017 May 30;114(22):5635-5640. doi: 10.1073/pnas.1620459114. Epub 2017 May 15. Proc Natl Acad Sci U S A. 2017. PMID: 28507156 Free PMC article.
-
Residues located in the primase domain of the bacteriophage T7 primase-helicase are essential for loading the hexameric complex onto DNA.J Biol Chem. 2022 Jun;298(6):101996. doi: 10.1016/j.jbc.2022.101996. Epub 2022 Apr 30. J Biol Chem. 2022. PMID: 35500649 Free PMC article.
References
-
- Kornberg A, Baker TA. DNA Replication. 2nd Ed. W.H. Freeman; New York: 1992. p. xiv.
-
- Hamdan SM, Richardson CC. Motors, switches, and contacts in the replisome. Annu Rev Biochem. 2009;78:205–243. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

