Imaging and Selective Elimination of Glioblastoma Stem Cells with Theranostic Near-Infrared-Labeled CD133-Specific Antibodies
- PMID: 27162556
- PMCID: PMC4860894
- DOI: 10.7150/thno.12890
Imaging and Selective Elimination of Glioblastoma Stem Cells with Theranostic Near-Infrared-Labeled CD133-Specific Antibodies
Abstract
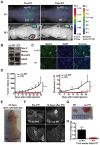
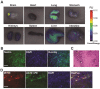
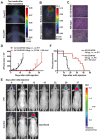
Near-infrared photoimmunotherapy (NIR-PIT), which employs monoclonal antibody (mAb)-phototoxic phthalocyanine dye IR700 conjugates, permits the specific, image-guided and spatiotemporally controlled elimination of tumor cells. Here, we report the highly efficient NIR-PIT of human tumor xenografts initiated from patient-derived cancer stem cells (CSCs). Using glioblastoma stem cells (GBM-SCs) expressing the prototypic CSC marker AC133/CD133, we also demonstrate here for the first time that NIR-PIT is highly effective against brain tumors. The intravenously injected theranostic AC133 mAb conjugate enabled the non-invasive detection of orthotopic gliomas by NIR fluorescence imaging, and reached AC133+ GBM-SCs at the invasive tumor front. AC133-targeted NIR-PIT induced the rapid cell death of AC133+ GBM-SCs and thereby strong shrinkage of both subcutaneous and invasively growing brain tumors. A single round of NIR-PIT extended the overall survival of mice with established orthotopic gliomas by more than a factor of two, even though the harmless NIR light was applied through the intact skull. Humanised versions of this theranostic agent may facilitate intraoperative imaging and histopathological evaluation of tumor borders and enable the highly specific and efficient eradication of CSCs.
Keywords: CD133; cancer stem cells; glioblastoma; near-infrared photoimmunotherapy.
Conflict of interest statement
Competing Interests: The authors have declared that no competing interest exists.
Figures



Similar articles
-
Effective Eradication of Glioblastoma Stem Cells by Local Application of an AC133/CD133-Specific T-cell-Engaging Antibody and CD8 T Cells.Cancer Res. 2015 Jun 1;75(11):2166-76. doi: 10.1158/0008-5472.CAN-14-2415. Epub 2015 Apr 3. Cancer Res. 2015. PMID: 25840983
-
A Novel Theranostic Combination of Near-infrared Fluorescence Imaging and Laser Irradiation Targeting c-KIT for Gastrointestinal Stromal Tumors.Theranostics. 2018 Mar 21;8(9):2313-2328. doi: 10.7150/thno.22027. eCollection 2018. Theranostics. 2018. PMID: 29721082 Free PMC article.
-
Neutrophil-Targeting Semiconducting Polymer Nanotheranostics for NIR-II Fluorescence Imaging-Guided Photothermal-NO-Immunotherapy of Orthotopic Glioblastoma.Adv Sci (Weinh). 2024 Oct;11(39):e2406750. doi: 10.1002/advs.202406750. Epub 2024 Aug 19. Adv Sci (Weinh). 2024. PMID: 39159216 Free PMC article.
-
Near-Infrared Photoimmunotherapy of Cancer.Acc Chem Res. 2019 Aug 20;52(8):2332-2339. doi: 10.1021/acs.accounts.9b00273. Epub 2019 Jul 23. Acc Chem Res. 2019. PMID: 31335117 Free PMC article. Review.
-
Inorganic and hybrid nanomaterials for NIR-II fluorescence imaging-guided therapy of Glioblastoma and perspectives.Theranostics. 2025 Apr 21;15(12):5616-5665. doi: 10.7150/thno.112204. eCollection 2025. Theranostics. 2025. PMID: 40365286 Free PMC article. Review.
Cited by
-
Nanomedicine-based immunotherapy for central nervous system disorders.Acta Pharmacol Sin. 2020 Jul;41(7):936-953. doi: 10.1038/s41401-020-0429-z. Epub 2020 May 28. Acta Pharmacol Sin. 2020. PMID: 32467570 Free PMC article. Review.
-
Near-infrared photoimmunotherapy of cancer: a new approach that kills cancer cells and enhances anti-cancer host immunity.Int Immunol. 2021 Jan 1;33(1):7-15. doi: 10.1093/intimm/dxaa037. Int Immunol. 2021. PMID: 32496557 Free PMC article.
-
EpCAM-targeted near-infrared photoimmunotherapy (NIR-PIT) for the treatment of breast cancer.Ann Med. 2025 Dec;57(1):2540599. doi: 10.1080/07853890.2025.2540599. Epub 2025 Aug 12. Ann Med. 2025. PMID: 40792441 Free PMC article.
-
Near-Infrared Photoimmunotherapy Using a Small Protein Mimetic for HER2-Overexpressing Breast Cancer.Int J Mol Sci. 2019 Nov 20;20(23):5835. doi: 10.3390/ijms20235835. Int J Mol Sci. 2019. PMID: 31757056 Free PMC article.
-
Combined-therapeutic strategies synergistically potentiate glioblastoma multiforme treatment via nanotechnology.Theranostics. 2020 Feb 10;10(7):3223-3239. doi: 10.7150/thno.40298. eCollection 2020. Theranostics. 2020. PMID: 32194864 Free PMC article. Review.
References
-
- Richards-Kortum R, Sevick-Muraca E. Quantitative optical spectroscopy for tissue diagnosis. Annu Rev Phys Chem. 1996;47:555–606. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

