KHF16 is a Leading Structure from Cimicifuga foetida that Suppresses Breast Cancer Partially by Inhibiting the NF-κB Signaling Pathway
- PMID: 27162557
- PMCID: PMC4860895
- DOI: 10.7150/thno.14694
KHF16 is a Leading Structure from Cimicifuga foetida that Suppresses Breast Cancer Partially by Inhibiting the NF-κB Signaling Pathway
Abstract
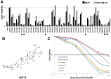
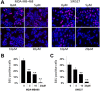
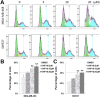
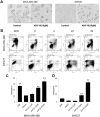
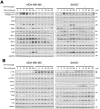
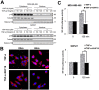
Triterpenoids extracted from Cimicifuga foetida have been reported to inhibit cancer by inducing cell cycle arrest and apoptosis. In this study, KHF16 (24-acetylisodahurinol-3-O-β-D-xylopyranoside), a cycloartane triterpenoid isolated from the rhizomes of C. foetida, showed potent anti-cancer activity in multiple ERα/PR/HER2 triple-negative breast cancer (TNBC) cell lines. KHF16 significantly induces cell cycle G2/M phase arrest and apoptosis in both MDA-MB-468 and SW527 TNBC cell lines. KHF16 reduces the expression levels of XIAP, Mcl-1, Survivin and Cyclin B1/D1 proteins. Importantly, KHF16 inhibits TNFα-induced IKKα/β phosphorylation, IKBα phosphorylation, p65 nuclear translocation and NF-κB downstream target gene expression, including XIAP, Mcl-1 and Survivin, in TNBC cells. These results suggest that KHF16 may inhibit TNBC by blocking the NF-κB signaling pathway in part.
Keywords: Apoptosis; Cell cycle; Cycloartane triterpenoid; KHF16; NF-κB.; Triple negative breast cancer.
Conflict of interest statement
Conflict of Interest: The authors have no conflicts of interest.
Figures

References
-
- Siegel R, Ma J, Zou Z, Jemal A. Cancer statistics, 2014. CA: a cancer journal for clinicians. 2014;64:9–29. - PubMed
-
- De Laurentiis M, Cianniello D, Caputo R, Stanzione B, Arpino G, Cinieri S. et al. Treatment of triple negative breast cancer (TNBC): current options and future perspectives. Cancer treatment reviews. 2010;36(Suppl 3):S80–6. - PubMed
-
- Fei F, Tang L, Di G, Wu J, Shao Z. Triple negative breast cancer (TNBC) patients diagnosed at different age present similar clinicopathological features, but different treatment and prognosis in Chinese population. Journal of geriatric oncology. 2014;5(Suppl 1):S9.
-
- Portt L, Norman G, Clapp C, Greenwood M, Greenwood MT. Anti-apoptosis and cell survival: a review. Biochimica et biophysica acta. 2011;1813:238–59. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

