Neuroendocrine hormone amylin in diabetes
- PMID: 27162583
- PMCID: PMC4856891
- DOI: 10.4239/wjd.v7.i9.189
Neuroendocrine hormone amylin in diabetes
Abstract
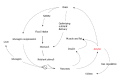
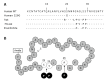
The neuroendocrine hormone amylin, also known as islet amyloid polypeptide, is co-localized, co-packaged and co-secreted with insulin from adult pancreatic islet β cells to maintain glucose homeostasis. Specifically, amylin reduces secretion of nutrient-stimulated glucagon, regulates blood pressure with an effect on renin-angiotensin system, and delays gastric emptying. The physiological actions of human amylin attribute to the conformational α-helix monomers whereas the misfolding instable oligomers may be detrimental to the islet β cells and further transform to β-sheet fibrils as amyloid deposits. No direct evidence proves that the amylin fibrils in amyloid deposits cause diabetes. Here we also have performed a systematic review of human amylin gene changes and reported the S20G mutation is minor in the development of diabetes. In addition to the metabolic effects, human amylin may modulate autoimmunity and innate inflammation through regulatory T cells to impact on both human type 1 and type 2 diabetes.
Keywords: Amylin; Diabetes; Neuroendocrine hormone.
Figures

References
-
- Westermark P, Wernstedt C, Wilander E, Sletten K. A novel peptide in the calcitonin gene related peptide family as an amyloid fibril protein in the endocrine pancreas. Biochem Bioph Res Co. 1986;140:827–831. - PubMed
-
- Clark A, Chargé SB, Badman MK, de Koning EJ. Islet amyloid in type 2 (non-insulin-dependent) diabetes. APMIS. 1996;104:12–18. - PubMed
-
- Clark A, Charge SB, Badman MK, MacArthur DA, de Koning EJ. Islet amyloid polypeptide: actions and role in the pathogenesis of diabetes. Biochem Soc Trans. 1996;24:594–599. - PubMed
-
- Kahn SE, Andrikopoulos S, Verchere CB. Islet amyloid: a long-recognized but underappreciated pathological feature of type 2 diabetes. Diabetes. 1999;48:241–253. - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources

