Pulmonary vascular morphology as an imaging biomarker in chronic thromboembolic pulmonary hypertension
- PMID: 27162616
- PMCID: PMC4860553
- DOI: 10.1086/685081
Pulmonary vascular morphology as an imaging biomarker in chronic thromboembolic pulmonary hypertension
Abstract
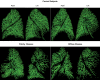
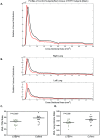
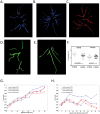
Patients with chronic thromboembolic pulmonary hypertension (CTEPH) have morphologic changes to the pulmonary vasculature. These include pruning of the distal vessels, dilation of the proximal vessels, and increased vascular tortuosity. Advances in image processing and computer vision enable objective detection and quantification of these processes in clinically acquired computed tomographic (CT) scans. Three-dimensional reconstructions of the pulmonary vasculature were created from the CT angiograms of 18 patients with CTEPH diagnosed using imaging and hemodynamics as well as 15 control patients referred to our Dyspnea Clinic and found to have no evidence of pulmonary vascular disease. Compared to controls, CTEPH patients exhibited greater pruning of the distal vasculature (median density of small-vessel volume: 2.7 [interquartile range (IQR): 2.5-3.0] vs. 3.2 [3.0-3.8]; P = 0.008), greater dilation of proximal arteries (median fraction of blood in large arteries: 0.35 [IQR: 0.30-0.41] vs. 0.23 [0.21-0.31]; P = 0.0005), and increased tortuosity in the pulmonary arterial tree (median: 4.92% [IQR: 4.85%-5.21%] vs. 4.63% [4.39%-4.92%]; P = 0.004). CTEPH was not associated with dilation of proximal veins or increased tortuosity in the venous system. Distal pruning of the vasculature was correlated with the cardiac index (R = 0.51, P = 0.04). Quantitative models derived from CT scans can be used to measure changes in vascular morphology previously described subjectively in CTEPH. These measurements are also correlated with invasive metrics of pulmonary hemodynamics, suggesting that they may be used to assess disease severity. Further work in a larger cohort may enable the use of such measures as a biomarker for diagnostic, phenotyping, and prognostic purposes.
Keywords: arterial; chronic thromboembolic pulmonary hypertension; computed tomography; tortuosity.
Figures


Similar articles
-
Quantification of Arterial and Venous Morphologic Markers in Pulmonary Arterial Hypertension Using CT Imaging.Chest. 2021 Dec;160(6):2220-2231. doi: 10.1016/j.chest.2021.06.069. Epub 2021 Jul 13. Chest. 2021. PMID: 34270966 Free PMC article.
-
Artificial intelligence-driven quantitative analysis of CT morphological differences between chronic thromboembolic pulmonary hypertension and chronic thromboembolic disease.Quant Imaging Med Surg. 2025 Feb 1;15(2):1101-1113. doi: 10.21037/qims-24-1301. Epub 2025 Jan 22. Quant Imaging Med Surg. 2025. PMID: 39995726 Free PMC article.
-
Recent progress in the diagnosis and management of chronic thromboembolic pulmonary hypertension.Respir Investig. 2013 Sep;51(3):134-46. doi: 10.1016/j.resinv.2013.02.005. Epub 2013 Apr 30. Respir Investig. 2013. PMID: 23978639 Review.
-
A multiscale model of vascular function in chronic thromboembolic pulmonary hypertension.Am J Physiol Heart Circ Physiol. 2021 Aug 1;321(2):H318-H338. doi: 10.1152/ajpheart.00086.2021. Epub 2021 Jun 18. Am J Physiol Heart Circ Physiol. 2021. PMID: 34142886 Free PMC article.
-
Chronic thromboembolic pulmonary hypertension (CTEPH) - potential role of multidetector-row CT (MD-CT) and MR imaging in the diagnosis and differential diagnosis of the disease.Rofo. 2014 Aug;186(8):751-61. doi: 10.1055/s-0034-1366425. Epub 2014 Apr 22. Rofo. 2014. PMID: 24756429 Review.
Cited by
-
Image-based scaling laws for somatic growth and pulmonary artery morphometry from infancy to adulthood.Am J Physiol Heart Circ Physiol. 2020 Aug 1;319(2):H432-H442. doi: 10.1152/ajpheart.00123.2020. Epub 2020 Jul 3. Am J Physiol Heart Circ Physiol. 2020. PMID: 32618514 Free PMC article.
-
The Importance of Topographical Recognition of Pulmonary Arteries in Diagnostics and Treatment of CTEPH, Based on an Analysis of a Dissected Case Model-A Pilot Study.Diagnostics (Basel). 2024 Aug 3;14(15):1684. doi: 10.3390/diagnostics14151684. Diagnostics (Basel). 2024. PMID: 39125560 Free PMC article.
-
Treatment Response Evaluation by Computed Tomography Pulmonary Vasculature Analysis in Patients With Chronic Thromboembolic Pulmonary Hypertension.Korean J Radiol. 2023 Apr;24(4):349-361. doi: 10.3348/kjr.2022.0675. Epub 2023 Mar 7. Korean J Radiol. 2023. PMID: 36907594 Free PMC article.
-
Automatic quantitative analysis of pulmonary vascular morphology in CT images.Med Phys. 2019 Sep;46(9):3985-3997. doi: 10.1002/mp.13659. Epub 2019 Jul 9. Med Phys. 2019. PMID: 31206181 Free PMC article.
-
Nanoparticle-Facilitated Gene Delivery in Congenital Pulmonary Vascular Disease: Roadmap for Other Forms of Pulmonary Hypertension.Circulation. 2021 Aug 17;144(7):556-558. doi: 10.1161/CIRCULATIONAHA.121.055345. Epub 2021 Aug 16. Circulation. 2021. PMID: 34398687 Free PMC article. No abstract available.
References
References Cited Only in the Appendix
-
- Xiao C, Staring M, Shamonin D, Reiber JH, Stolk J, Stoel BC. A strain energy filter for 3D vessel enhancement with application to pulmonary CT images. Med Image Anal 2011;15(1):112–124. - PubMed
-
- Kruskal JB Jr. On the shortest spanning subtree of a graph and the traveling salesman problem. Proc Am Math Soc 1956;7(1):48–50.
References
-
- Morris TA. Why acute pulmonary embolism becomes chronic thromboembolic pulmonary hypertension: clinical and genetic insights. Curr Opin Pulm Med 2013;19(5):422–429. - PubMed
-
- Delcroix M, Vonk Noordegraaf A, Fadel E, Lang I, Simonneau G, Naeije R. Vascular and right ventricular remodelling in chronic thromboembolic pulmonary hypertension. Eur Respir J 2013;41(1):224–232. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

