Methods and clinical development of adenovirus-vectored vaccines against mucosal pathogens
- PMID: 27162933
- PMCID: PMC4847555
- DOI: 10.1038/mtm.2016.30
Methods and clinical development of adenovirus-vectored vaccines against mucosal pathogens
Abstract
Adenoviruses represent the most widely used viral-vectored platform for vaccine design, showing a great potential in the fight against intracellular infectious diseases to which either there is a lack of effective vaccines or the traditional vaccination strategy is suboptimal. The extensive understanding of the molecular biology of adenoviruses has made the new technologies and reagents available to efficient generation of adenoviral-vectored vaccines for both preclinical and clinical evaluation. The novel adenoviral vectors including nonhuman adenoviral vectors have emerged to be the further improved vectors for vaccine design. In this review, we discuss the latest adenoviral technologies and their utilization in vaccine development. We particularly focus on the application of adenoviral-vectored vaccines in mucosal immunization strategies against mucosal pathogens including Mycobacterium tuberculosis, flu virus, and human immunodeficiency virus.
Figures
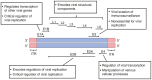
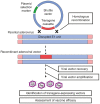
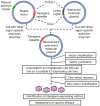
References
-
- Volpers, C and Kochanek, S (2004). Adenoviral vectors for gene transfer and therapy. J Gene Med 6 (suppl. 1): S164–S171. - PubMed
-
- Majhen, D, Calderon, H, Chandra, N, Fajardo, CA, Rajan, A, Alemany, R et al. (2014). Adenovirus-based vaccines for fighting infectious diseases and cancer: progress in the field. Hum Gene Ther 25: 301–317. - PubMed
-
- Sheridan, C (2011). Gene therapy finds its niche. Nat Biotechnol 29: 121–128. - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources

