Chimeric antigen receptor T-cell therapy for solid tumors
- PMID: 27162934
- PMCID: PMC4849432
- DOI: 10.1038/mto.2016.6
Chimeric antigen receptor T-cell therapy for solid tumors
Abstract
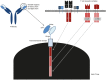
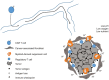
Chimeric antigen receptor (CAR) T cells are engineered constructs composed of synthetic receptors that direct T cells to surface antigens for subsequent elimination. Many CAR constructs are also manufactured with elements that augment T-cell persistence and activity. To date, CAR T cells have demonstrated tremendous success in eradicating hematological malignancies (e.g., CD19 CARs in leukemias). This success is not yet extrapolated to solid tumors, and the reasons for this are being actively investigated. Here in this mini-review, we discuss some of the key hurdles encountered by CAR T cells in the solid tumor microenvironment.
Figures
References
-
- Willemsen, RA, Debets, R, Hart, E, Hoogenboom, HR, Bolhuis, RL and Chames, P (2001). A phage display selected fab fragment with MHC class I-restricted specificity for MAGE-A1 allows for retargeting of primary human T lymphocytes. Gene Ther 8: 1601–1608. - PubMed
-
- Gill, S, Maus, MV and Porter, DL (2015). Chimeric antigen receptor T cell therapy: 25 years in the making. Blood Rev (Epub ahead of print). - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

