Synthesis, Properties, and Design Principles of Donor-Acceptor Nanohoops
- PMID: 27162989
- PMCID: PMC4827663
- DOI: 10.1021/acscentsci.5b00269
Synthesis, Properties, and Design Principles of Donor-Acceptor Nanohoops
Abstract
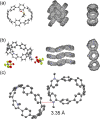
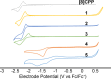
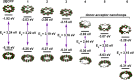
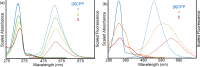
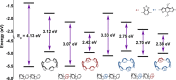
We have synthesized a series of aza[8]cycloparaphenylenes containing one, two, and three nitrogens to probe the impact of nitrogen doping on optoelectronic properties and solid state packing. Alkylation of these azananohoops afforded the first donor-acceptor nanohoops where the phenylene backbone acts as the donor and the pyridinium units act as the acceptor. The impact on the optoelectronic properties was then studied experimentally and computationally to provide new insight into the effect of functionalization on nanohoops properties.
Conflict of interest statement
The authors declare no competing financial interest.
Figures




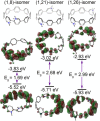
References
-
- McQuade D. T.; Pullen A. E.; Swager T. M. Conjugated Polymer-Based Chemical Sensors. Chem. Rev. 2000, 100 (7), 2537–2574. 10.1021/cr9801014. - DOI - PubMed
- Izuhara D.; Swager T. M. Bispyridinium-phenylene-based copolymers: low band gap n-type alternating copolymers. J. Mater. Chem. 2011, 21 (11), 3579–3584. 10.1039/c0jm02530e. - DOI
- Izuhara D.; Swager T. M. Poly(Pyridinium Phenylene)s: Water-Soluble N-Type Polymers. J. Am. Chem. Soc. 2009, 131 (49), 17724–17725. 10.1021/ja906513u. - DOI - PubMed
-
- Golder M. R.; Jasti R. Syntheses of the Smallest Carbon Nanohoops and the Emergence of Unique Physical Phenomena. Acc. Chem. Res. 2015, 48 (3), 557–566. 10.1021/ar5004253. - DOI - PubMed
- Darzi E. R.; Jasti R. The dynamic, size-dependent properties of [5]-[12]cycloparaphenylenes. Chem. Soc. Rev. 2015, 44, 6401.10.1039/C5CS00143A. - DOI - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources

