Molecular Origins of Mesoscale Ordering in a Metalloamphiphile Phase
- PMID: 27163014
- PMCID: PMC4827664
- DOI: 10.1021/acscentsci.5b00306
Molecular Origins of Mesoscale Ordering in a Metalloamphiphile Phase
Abstract
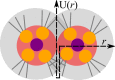
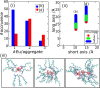
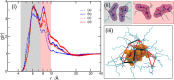
Controlling the assembly of soft and deformable molecular aggregates into mesoscale structures is essential for understanding and developing a broad range of processes including rare earth extraction and cleaning of water, as well as for developing materials with unique properties. By combined synchrotron small- and wide-angle X-ray scattering with large-scale atomistic molecular dynamics simulations we analyze here a metalloamphiphile-oil solution that organizes on multiple length scales. The molecules associate into aggregates, and aggregates flocculate into meso-ordered phases. Our study demonstrates that dipolar interactions, centered on the amphiphile headgroup, bridge ionic aggregate cores and drive aggregate flocculation. By identifying specific intermolecular interactions that drive mesoscale ordering in solution, we bridge two different length scales that are classically addressed separately. Our results highlight the importance of individual intermolecular interactions in driving mesoscale ordering.
Conflict of interest statement
The authors declare no competing financial interest.
Figures





Similar articles
-
Mesoscale texture of cement hydrates.Proc Natl Acad Sci U S A. 2016 Feb 23;113(8):2029-34. doi: 10.1073/pnas.1520487113. Epub 2016 Feb 8. Proc Natl Acad Sci U S A. 2016. PMID: 26858450 Free PMC article.
-
Liquid worm-like and proto-micelles: water solubilization in amphiphile-oil solutions.Phys Chem Chem Phys. 2018 May 9;20(18):12908-12915. doi: 10.1039/c8cp00600h. Phys Chem Chem Phys. 2018. PMID: 29700533
-
Two-Dimensional Mesoscale-Ordered Conducting Polymers.Angew Chem Int Ed Engl. 2016 Sep 26;55(40):12516-21. doi: 10.1002/anie.201606988. Epub 2016 Sep 7. Angew Chem Int Ed Engl. 2016. PMID: 27603275
-
Twisted Structures in Natural and Bioinspired Molecules: Self-Assembly and Propagation of Chirality Across Multiple Length Scales.ACS Omega. 2023 May 10;8(20):17350-17361. doi: 10.1021/acsomega.3c01822. eCollection 2023 May 23. ACS Omega. 2023. PMID: 37251126 Free PMC article. Review.
-
Understanding dislocation mechanics at the mesoscale using phase field dislocation dynamics.Philos Trans A Math Phys Eng Sci. 2016 Apr 28;374(2066):20150166. doi: 10.1098/rsta.2015.0166. Philos Trans A Math Phys Eng Sci. 2016. PMID: 27002063 Free PMC article. Review.
Cited by
-
How to explain microemulsions formed by solvent mixtures without conventional surfactants.Proc Natl Acad Sci U S A. 2016 Apr 19;113(16):4260-5. doi: 10.1073/pnas.1515708113. Epub 2016 Apr 1. Proc Natl Acad Sci U S A. 2016. PMID: 27044068 Free PMC article.
-
Hierarchical Aggregation in a Complex Fluid─The Role of Isomeric Interconversion.J Phys Chem B. 2023 Mar 9;127(9):2052-2065. doi: 10.1021/acs.jpcb.2c07527. Epub 2023 Feb 23. J Phys Chem B. 2023. PMID: 36821599 Free PMC article.
-
A Telescoping View of Solute Architectures in a Complex Fluid System.ACS Cent Sci. 2019 Jan 23;5(1):85-96. doi: 10.1021/acscentsci.8b00669. Epub 2018 Dec 31. ACS Cent Sci. 2019. PMID: 30693328 Free PMC article.
-
Colloidal Model for the Prediction of the Extraction of Rare Earths Assisted by the Acidic Extractant.Langmuir. 2019 Feb 26;35(8):3215-3230. doi: 10.1021/acs.langmuir.8b03846. Epub 2019 Feb 15. Langmuir. 2019. PMID: 30673246 Free PMC article.
-
Homopolymer self-assembly of poly(propylene sulfone) hydrogels via dynamic noncovalent sulfone-sulfone bonding.Nat Commun. 2020 Sep 29;11(1):4896. doi: 10.1038/s41467-020-18657-5. Nat Commun. 2020. PMID: 32994414 Free PMC article.
References
LinkOut - more resources
Full Text Sources
Other Literature Sources

