An Expeditious Synthesis of Sialic Acid Derivatives by Copper(I)-Catalyzed Stereodivergent Propargylation of Unprotected Aldoses
- PMID: 27163022
- PMCID: PMC4827533
- DOI: 10.1021/acscentsci.5b00360
An Expeditious Synthesis of Sialic Acid Derivatives by Copper(I)-Catalyzed Stereodivergent Propargylation of Unprotected Aldoses
Abstract
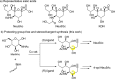
We developed a copper(I)-catalyzed stereodivergent anomeric propargylation of unprotected aldoses as a facile synthetic pathway to a broad variety of sialic acid derivatives. The soft allenylcopper(I) species, catalytically generated from stable allenylboronic acid pinacolate (2), is unusually inert to protonolysis by the multiple hydroxy groups of the substrates and thereby functions as a carbon nucleophile. The key additive B(OMe)3 facilitated ring-opening of the nonelectrophilic cyclic hemiacetal forms of aldoses to the reactive aldehyde forms. The chirality of the catalyst, and not the internal stereogenic centers of substrates, predominantly controlled the stereochemistry of the propargylation step; i.e., the diastereoselectivity was switched simply by changing the catalyst chirality. This is the first nonenzyme catalyst-controlled stereodivergent C-C bond elongation at the anomeric center of unprotected aldoses, which contain multiple protic functional groups and stereogenic centers. The propargylation products can be expeditiously transformed into naturally occurring and synthetic sialic acid derivatives in a simple three-step sequence. This synthetic method, which requires no protecting groups, can be performed on a gram-scale and thus offers general and practical access to various sialic acid derivatives from unprotected aldoses.
Conflict of interest statement
The authors declare no competing financial interest.
Figures


Similar articles
-
Copper(I)-Catalyzed Stereodivergent Propargylation of N-Acetyl Mannosamine for Protecting Group Minimal Synthesis of C3-Substituted Sialic Acids.J Org Chem. 2019 Sep 6;84(17):10615-10628. doi: 10.1021/acs.joc.9b00887. Epub 2019 Aug 22. J Org Chem. 2019. PMID: 31379168
-
Catalytic anomeric aminoalkynylation of unprotected aldoses.Org Lett. 2013 Aug 16;15(16):4130-3. doi: 10.1021/ol401810b. Epub 2013 Jul 31. Org Lett. 2013. PMID: 23901780
-
Development of Copper-Catalyzed Chemoselective Reactions.Chem Pharm Bull (Tokyo). 2020;68(5):405-420. doi: 10.1248/cpb.c19-00739. Chem Pharm Bull (Tokyo). 2020. PMID: 32378539 Review.
-
Beyond Protecting Groups in Metal Catalyzed C-C Coupling: Direct Anomeric Propargylation of Aldoses.ACS Cent Sci. 2016 Jan 27;2(1):12-3. doi: 10.1021/acscentsci.6b00002. Epub 2016 Jan 15. ACS Cent Sci. 2016. PMID: 27163021 Free PMC article. No abstract available.
-
Copper-catalyzed divergent kinetic resolution of racemic allylic substrates.Chirality. 2011 Oct;23(9):703-10. doi: 10.1002/chir.20980. Epub 2011 Aug 12. Chirality. 2011. PMID: 21837639 Review.
Cited by
-
Enantioselective copper catalysed, direct functionalisation of allenes via allyl copper intermediates.Chem Sci. 2017 Aug 1;8(8):5240-5247. doi: 10.1039/c7sc01968h. Epub 2017 Jun 8. Chem Sci. 2017. PMID: 28959423 Free PMC article. Review.
-
Regioselective Manipulation of GlcNAc Provides Allosamine, Lividosamine, and Related Compounds.J Org Chem. 2019 Jan 18;84(2):516-525. doi: 10.1021/acs.joc.8b01949. Epub 2019 Jan 2. J Org Chem. 2019. PMID: 30569712 Free PMC article.
-
Thermal, Spectroscopic, and Crystallographic Analysis of Mannose-Derived Linear Polyols.Cryst Growth Des. 2018 May 2;18(5):3151-3160. doi: 10.1021/acs.cgd.8b00263. Epub 2018 Apr 9. Cryst Growth Des. 2018. PMID: 30258305 Free PMC article.
-
Enantioselective Generation of Adjacent Stereocenters in a Copper-Catalyzed Three-Component Coupling of Imines, Allenes, and Diboranes.Angew Chem Int Ed Engl. 2016 Sep 19;55(39):11912-6. doi: 10.1002/anie.201606710. Epub 2016 Aug 19. Angew Chem Int Ed Engl. 2016. PMID: 27539673 Free PMC article.
-
Catalytic Enantioselective Carbonyl Propargylation Beyond Preformed Carbanions: Reductive Coupling and Hydrogen Auto-Transfer.ChemCatChem. 2019 Jan 9;11(1):324-332. doi: 10.1002/cctc.201801121. Epub 2018 Aug 7. ChemCatChem. 2019. PMID: 31588251 Free PMC article.
References
-
-
For selected examples of catalytic asymmetric nucleophilic alkylation of carbonyl compounds in the presence of protic functional groups, see
- Sakai M.; Ueda M.; Miyaura N. Rhodium-catalyzed addition of organoboronic acids to aldehydes. Angew. Chem., Int. Ed. 1998, 37, 3279–3281. 10.1002/(SICI)1521-3773(19981217)37:23<3279::AID-ANIE3279>3.0.CO;2-M. - DOI - PubMed
- Loh T.-P.; Zhou J.-R. A catalytic enantioselective allylation reaction of aldehydes in an aqueous medium. Tetrahedron Lett. 2000, 41, 5261–5264. 10.1016/S0040-4039(00)00801-7. - DOI
- Zhu S.-F.; Yang Y.; Wang L.-X.; Liu B.; Zhou Q.-L. Synthesis and application of chiral spiro phospholane ligand in Pd-catalyzed asymmetric allylation of aldehydes with allylic alcohols. Org. Lett. 2005, 7, 2333–2335. 10.1021/ol050556x. - DOI - PubMed
- Kobayashi S.; Endo T.; Yoshino T.; Schneider U.; Ueno M. Allylation reactions of aldehydes with allylboronates in aqueous media: unique reactivity and selectivity that are only observed in the presence of water. Chem. - Asian J. 2013, 8, 2033–2045. 10.1002/asia.201300440. - DOI - PubMed
-
-
-
For a recent review and selected reports, see
- Dechert-Schmitt A.-M. R.; Schmitt D. C.; Gao X.; Itoh T.; Krische M. J. Polyketide construction via hydrohydroxyalkylation and related alcohol C-H functionalizations: reinventing the chemistry of carbonyl addition. Nat. Prod. Rep. 2014, 31, 504–513. 10.1039/c3np70076c. - DOI - PMC - PubMed
- Tsutsumi R.; Hong S.; Krische M. J. Diastereo- and enantioselective iridium catalyzed carbonyl (α-cyclopropyl)allylation via transfer hydrogenation. Chem. - Eur. J. 2015, 21, 12903–12907. 10.1002/chem.201502499. - DOI - PMC - PubMed
- Liang T.; Nguyen K. D.; Zhang W.; Krische M. J. Enantioselective ruthenium-catalyzed carbonyl allylation via alkyne-alcohol C-C bond-forming transfer hydrogenation: allene hydrometalation vs oxidative coupling. J. Am. Chem. Soc. 2015, 137, 3161–3164. 10.1021/jacs.5b00747. - DOI - PMC - PubMed
-
LinkOut - more resources
Full Text Sources
Other Literature Sources

