Generation of stem cell-derived β-cells from patients with type 1 diabetes
- PMID: 27163171
- PMCID: PMC4866045
- DOI: 10.1038/ncomms11463
Generation of stem cell-derived β-cells from patients with type 1 diabetes
Erratum in
-
Corrigendum: Generation of stem cell-derived β-cells from patients with type 1 diabetes.Nat Commun. 2016 Aug 4;7:12379. doi: 10.1038/ncomms12379. Nat Commun. 2016. PMID: 27487970 Free PMC article. No abstract available.
Abstract
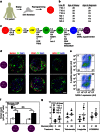
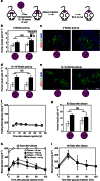
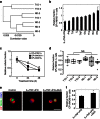
We recently reported the scalable in vitro production of functional stem cell-derived β-cells (SC-β cells). Here we extend this approach to generate the first SC-β cells from type 1 diabetic patients (T1D). β-cells are destroyed during T1D disease progression, making it difficult to extensively study them in the past. These T1D SC-β cells express β-cell markers, respond to glucose both in vitro and in vivo, prevent alloxan-induced diabetes in mice and respond to anti-diabetic drugs. Furthermore, we use an in vitro disease model to demonstrate the cells respond to different forms of β-cell stress. Using these assays, we find no major differences in T1D SC-β cells compared with SC-β cells derived from non-diabetic patients. These results show that T1D SC-β cells could potentially be used for the treatment of diabetes, drug screening and the study of β-cell biology.
Conflict of interest statement
J.R.M., M.G., F.W.P. and D.A.M. are inventors on patent applications for content described in this manuscript. F.W.P. is an employee at Semma Therapeutics.
Figures
References
-
- Santin I. & Eizirik D. L. Candidate genes for type 1 diabetes modulate pancreatic islet inflammation and beta-cell apoptosis. Diabetes Obes. Metab. 15, 71–81 (2013). - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

