Lateral flow urine lipoarabinomannan assay for detecting active tuberculosis in HIV-positive adults
- PMID: 27163343
- PMCID: PMC4916932
- DOI: 10.1002/14651858.CD011420.pub2
Lateral flow urine lipoarabinomannan assay for detecting active tuberculosis in HIV-positive adults
Abstract
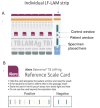
Background: Rapid detection of tuberculosis (TB) among people living with human immunodeficiency virus (HIV) is a global health priority. HIV-associated TB may have different clinical presentations and is challenging to diagnose. Conventional sputum tests have reduced sensitivity in HIV-positive individuals, who have higher rates of extrapulmonary TB compared with HIV-negative individuals. The lateral flow urine lipoarabinomannan assay (LF-LAM) is a new, commercially available point-of-care test that detects lipoarabinomannan (LAM), a lipopolysaccharide present in mycobacterial cell walls, in people with active TB disease.
Objectives: To assess the accuracy of LF-LAM for the diagnosis of active TB disease in HIV-positive adults who have signs and symptoms suggestive of TB (TB diagnosis).To assess the accuracy of LF-LAM as a screening test for active TB disease in HIV-positive adults irrespective of signs and symptoms suggestive of TB (TB screening).
Search methods: We searched the following databases without language restriction on 5 February 2015: the Cochrane Infectious Diseases Group Specialized Register; MEDLINE (PubMed,1966); EMBASE (OVID, from 1980); Science Citation Index Expanded (SCI-EXPANDED, from 1900), Conference Proceedings Citation Index-Science (CPCI-S, from 1900), and BIOSIS Previews (from 1926) (all three using the Web of Science platform; MEDION; LILACS (BIREME, from 1982); SCOPUS (from 1995); the metaRegister of Controlled Trials (mRCT); the search portal of the World Health Organization International Clinical Trials Registry Platform (WHO ICTRP); and ProQuest Dissertations & Theses A&l (from 1861).
Selection criteria: Eligible study types included randomized controlled trials, cross-sectional studies, and cohort studies that determined LF-LAM accuracy for TB against a microbiological reference standard (culture or nucleic acid amplification test from any body site). A higher quality reference standard was one in which two or more specimen types were evaluated for TB, and a lower quality reference standard was one in which only one specimen type was evaluated for TB. Participants were HIV-positive people aged 15 years and older.
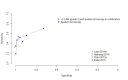
Data collection and analysis: Two review authors independently extracted data from each included study using a standardized form. We appraised the quality of studies using the Quality Assessment of Diagnostic Accuracy Studies-2 (QUADAS-2) tool. We evaluated the test at two different cut-offs: (grade 1 or 2, based on the reference card scale of five intensity bands). Most analyses used grade 2, the manufacturer's currently recommended cut-off for positivity. We carried out meta-analyses to estimate pooled sensitivity and specificity using a bivariate random-effects model and estimated the models using a Bayesian approach. We determined accuracy of LF-LAM combined with sputum microscopy or Xpert® MTB/RIF. In addition, we explored the influence of CD4 count on the accuracy estimates. We assessed the quality of the evidence using the Grading of Recommendations Assessment, Development and Evaluation (GRADE) approach.
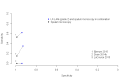
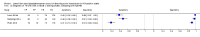
Main results: We included 12 studies: six studies evaluated LF-LAM for TB diagnosis and six studies evaluated the test for TB screening. All studies were cross-sectional or cohort studies. Studies for TB diagnosis were largely conducted among inpatients (median CD4 range 71 to 210 cells per µL) and studies for TB screening were largely conducted among outpatients (median CD4 range 127 to 437 cells per µL). All studies were conducted in low- or middle-income countries. Only two studies for TB diagnosis (33%) and one study for TB screening (17%) used a higher quality reference standard.LF-LAM for TB diagnosis (grade 2 cut-off): meta-analyses showed median pooled sensitivity and specificity (95% credible interval (CrI)) of 45% (29% to 63%) and 92% (80% to 97%), (five studies, 2313 participants, 35% with TB, low quality evidence). The pooled sensitivity of a combination of LF-LAM and sputum microscopy (either test positive) was 59% (47% to 70%), which represented a 19% (4% to 36%) increase over sputum microscopy alone, while the pooled specificity was 92% (73% to 97%), which represented a 6% (1% to 24%) decrease from sputum microscopy alone (four studies, 1876 participants, 38% with TB). The pooled sensitivity of a combination of LF-LAM and sputum Xpert® MTB/RIF (either test positive) was 75% (61% to 87%) and represented a 13% (1% to 37%) increase over Xpert® MTB/RIF alone. The pooled specificity was 93% (81% to 97%) and represented a 4% (1% to 16%) decrease from Xpert® MTB/RIF alone (three studies, 909 participants, 36% with TB). Pooled sensitivity and specificity of LF-LAM were 56% (41% to 70%) and 90% (81% to 95%) in participants with a CD4 count of less than or equal to 100 cells per µL (five studies, 859 participants, 47% with TB) versus 26% (16% to 46%) and 92% (78% to 97%) in participants with a CD4 count greater than 100 cells per µL (five studies, 1410 participants, 30% with TB).LF-LAM for TB screening (grade 2 cut-off): for individual studies, sensitivity estimates (95% CrI) were 44% (30% to 58%), 28% (16% to 42%), and 0% (0% to 71%) and corresponding specificity estimates were 95% (92% to 97%), 94% (90% to 97%), and 95% (92% to 97%) (three studies, 1055 participants, 11% with TB, very low quality evidence). There were limited data for additional analyses.The main limitations of the review were the use of a lower quality reference standard in most included studies, and the small number of studies and participants included in the analyses. The results should, therefore, be interpreted with caution.
Authors' conclusions: We found that LF-LAM has low sensitivity to detect TB in adults living with HIV whether the test is used for diagnosis or screening. For TB diagnosis, the combination of LF-LAM with sputum microscopy suggests an increase in sensitivity for TB compared to either test alone, but with a decrease in specificity. In HIV-positive individuals with low CD4 counts who are seriously ill, LF-LAM may help with the diagnosis of TB.
Conflict of interest statement
Development of the systematic review was in part made possible with financial support from the United States Agency for International Development (USAID) administered by the World Health Organization (WHO) Global TB Programme,Switzerland. KRS, MS, CH, and ZYW received funding to carry out the review from USAID. KRS served as Co‐ordinator of the Evidence Synthesis and Policy Subgroup of Stop TB Partnership’s New Diagnostics Working Group through 2015. SDL has received Wellcome Trust grant funds to conduct primary research evaluating diagnostic tests using this product. SDL published primary research that was included in the review and provided intellectual input to the review, but did not participate directly in the application of the inclusion criteria, 'Risk of bias' assessment, or data extraction. CMD is employed by FIND, a Swiss non‐profit organization. FIND provided funding for an initial assessment of data available for the review. KRS, MS, and ZYW received funding from FIND for an initial assessment. We presented the findings from the initial assessment at the Union World Conference on Lung Health, Barcelona, October 2014. MS is supported through an National Institute of Health (NIH) K23 grant (K23AI089259). ND has no declarations of interest to declare. The review authors have no financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the review apart from those disclosed.
Figures




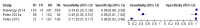





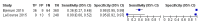










































Update of
References
References to studies included in this review
-
- Andrews B, Muchemwa L, Lakhi S, Bwalya M, Mabula C, Chipii G, Heimburger DC, Bernard GR. Validation of a clinical prediction score and performance of urine lipoarabinomannan test for detecting tuberculosis bacteremia in HIV‐positive patients with severe sepsis. American Thoracic Society. 2014:A2559.
-
- Balcha TT, Winqvist N, Sturegård E, Skogmar S, Reepalu A, Jemal ZH, et al. Detection of lipoarabinomannan in urine for identification of active tuberculosis among HIV‐positive adults in Ethiopian health centres. Tropical Medicine & International Health 2014;19(6):734‐42. - PubMed
References to studies excluded from this review
-
- Agha MA, El‐Helbawy RH, El‐Helbawy NG, El‐Sheak NM. Utility of quantitative analysis of urine lipoarabinomannan in the diagnosis of tuberculosis. Egyptian Journal of Chest Diseases and Tuberculosis 2013;62(3):401‐7.
-
- Amoudy HA, Al‐Asmer ABH, Abul AT, Mustafa AS. Evaluation of complex and defined antigens of Mycobacterium tuberculosis in an IgG‐specific ELISA for the diagnosis of tuberculosis. Medical Principles and Practice 1997;6(2):103‐9.
-
- Boehme C, Molokova E, Minja F, Geis S, Loscher T, Maboko L, et al. Detection of mycobacterial lipoarabinomannan with an antigen‐capture ELISA in unprocessed urine of Tanzanian patients with suspected tuberculosis. Transactions of the Royal Society of Tropical Medicine and Hygiene 2005;99(12):893‐900. - PubMed
-
- Boyles TH. The pros and cons of urinary lipoarabinomannan testing. AIDS 2012;26(17):2263‐4; author reply 2264‐5. - PubMed
References to studies awaiting assessment
-
- NCT01990274. The utility of intensified case finding combined with a package of novel TB diagnostics performed at community‐based‐clinics in South Africa – a multi‐centric prospective cohort study (XACT study). https://clinicaltrials.gov/ct2/show/NCT01990274?term=xact&rank=2 (accessed 04 September 2015).
References to ongoing studies
-
- Grant A. Xpert MTB/Rif for people attending HIV care: an interventional cohort study to guide rational implementation (XPHACTOR). http://www.google.com/url?sa=t&rct=j&q=&esrc=s&source=web&cd=10&ved=0ahU... (accessed 4 April 2016).
Additional references
-
- Alere. Alere DetermineTM TB LAM Ag Product Information. http://www.alere.com/ww/en/product‐details/determine‐tb‐lam.html (accessed 16 November 2014).
-
- Bartlett JG. Tuberculosis and HIV infection: partners in human tragedy. Journal of Infectious Diseases 2007;196(Suppl 1):S124‐5. - PubMed
-
- Batz HG, Cooke GS, Reid SD. Towards lab‐free tuberculosis diagnosis. August 2011. http://www.msfaccess.org/sites/default/files/MSF_assets/TB/Docs/TB_Repor.... London: Imperial College, (accessed 16 November 2014).
-
- Cain KP, McCarthy KD, Heilig CM, Monkongdee P, Tasaneeyapan T, Kanara N, et al. An algorithm for tuberculosis screening and diagnosis in people with HIV. New England Journal of Medicine 2010;362(8):707‐16. - PubMed
References to other published versions of this review
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

