Calcineurin Orchestrates Lateral Transfer of Aspergillus fumigatus during Macrophage Cell Death
- PMID: 27163634
- PMCID: PMC5114448
- DOI: 10.1164/rccm.201601-0070OC
Calcineurin Orchestrates Lateral Transfer of Aspergillus fumigatus during Macrophage Cell Death
Abstract
Rationale: Pulmonary aspergillosis is a lethal mold infection in the immunocompromised host. Understanding initial control of infection and how this is altered in the immunocompromised host are key goals for comprehension of the pathogenesis of pulmonary aspergillosis.
Objectives: To characterize the outcome of human macrophage infection with Aspergillus fumigatus and how this is altered in transplant recipients on calcineurin inhibitor immunosuppressants.
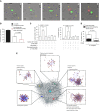
Methods: We defined the outcome of human macrophage infection with A. fumigatus, as well as the impact of calcineurin inhibitors, through a combination of single-cell fluorescence imaging, transcriptomics, proteomics, and in vivo studies.
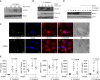
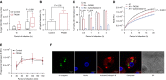
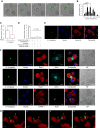
Measurements and main results: Macrophage phagocytosis of A. fumigatus enabled control of 90% of fungal germination. However, fungal germination in the late phagosome led to macrophage necrosis. During programmed necroptosis, we observed frequent cell-cell transfer of A. fumigatus between macrophages, which assists subsequent control of germination in recipient macrophages. Lateral transfer occurred through actin-dependent exocytosis of the late endosome in a vasodilator-stimulated phosphoprotein envelope. Its relevance to the control of fungal germination was also shown by direct visualization in our zebrafish aspergillosis model in vivo. The calcineurin inhibitor FK506 (tacrolimus) reduced cell death and lateral transfer in vitro by 50%. This resulted in uncontrolled fungal germination in macrophages and also resulted in hyphal escape.
Conclusions: These observations identify programmed, necrosis-dependent lateral transfer of A. fumigatus between macrophages as an important host strategy for controlling fungal germination. This process is critically dependent on calcineurin. Our studies provide fundamental insights into the pathogenesis of pulmonary aspergillosis in the immunocompromised host.
Keywords: Aspergillus; calcineurin; macrophage; necrosis; pulmonary fungal diseases.
Figures
Similar articles
-
Phagocytosis-dependent activation of a TLR9-BTK-calcineurin-NFAT pathway co-ordinates innate immunity to Aspergillus fumigatus.EMBO Mol Med. 2015 Mar;7(3):240-58. doi: 10.15252/emmm.201404556. EMBO Mol Med. 2015. PMID: 25637383 Free PMC article.
-
Macrophages inhibit extracellular hyphal growth of A. fumigatus through Rac2 GTPase signaling.Infect Immun. 2024 Feb 13;92(2):e0038023. doi: 10.1128/iai.00380-23. Epub 2024 Jan 3. Infect Immun. 2024. PMID: 38168666 Free PMC article.
-
Persistence versus escape: Aspergillus terreus and Aspergillus fumigatus employ different strategies during interactions with macrophages.PLoS One. 2012;7(2):e31223. doi: 10.1371/journal.pone.0031223. Epub 2012 Feb 3. PLoS One. 2012. PMID: 22319619 Free PMC article.
-
Pulmonary defense mechanisms against opportunistic fungal pathogens.Immunol Ser. 1989;47:243-71. Immunol Ser. 1989. PMID: 2490078 Review.
-
Calcineurin Orchestrates Hyphal Growth, Septation, Drug Resistance and Pathogenesis of Aspergillus fumigatus: Where Do We Go from Here?Pathogens. 2015 Dec 16;4(4):883-93. doi: 10.3390/pathogens4040883. Pathogens. 2015. PMID: 26694470 Free PMC article. Review.
Cited by
-
The Diverse Roles of Phagocytes During Bacterial and Fungal Infections and Sterile Inflammation: Lessons From Zebrafish.Front Immunol. 2020 Jun 5;11:1094. doi: 10.3389/fimmu.2020.01094. eCollection 2020. Front Immunol. 2020. PMID: 32582182 Free PMC article. Review.
-
Types of necroinflammation, the effect of cell death modalities on sterile inflammation.Cell Death Dis. 2022 May 2;13(5):423. doi: 10.1038/s41419-022-04883-w. Cell Death Dis. 2022. PMID: 35501340 Free PMC article. Review.
-
Stimulating the autophagic-lysosomal axis enhances host defense against fungal infection in a zebrafish model of invasive Aspergillosis.Autophagy. 2023 Jan;19(1):324-337. doi: 10.1080/15548627.2022.2090727. Epub 2022 Jul 1. Autophagy. 2023. PMID: 35775203 Free PMC article.
-
Infection of Zebrafish Larvae with Aspergillus Spores for Analysis of Host-Pathogen Interactions.J Vis Exp. 2020 May 16;(159):10.3791/61165. doi: 10.3791/61165. J Vis Exp. 2020. PMID: 32478760 Free PMC article.
-
Illuminating Macrophage Contributions to Host-Pathogen Interactions In Vivo: the Power of Zebrafish.Infect Immun. 2020 Jun 22;88(7):e00906-19. doi: 10.1128/IAI.00906-19. Print 2020 Jun 22. Infect Immun. 2020. PMID: 32179583 Free PMC article. Review.
References
-
- Chen GH, Teitz-Tennenbaum S, Neal LM, Murdock BJ, Malachowski AN, Dils AJ, Olszewski MA, Osterholzer JJ. Local GM-CSF-dependent differentiation and activation of pulmonary dendritic cells and macrophages protect against progressive cryptococcal lung infection in mice. J Immunol. 2016;196:1810–1821. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

