Interferon Gamma and Contact-dependent Cytotoxicity Are Each Rate Limiting for Natural Killer Cell-Mediated Antibody-dependent Chronic Rejection
- PMID: 27163757
- PMCID: PMC5083186
- DOI: 10.1111/ajt.13865
Interferon Gamma and Contact-dependent Cytotoxicity Are Each Rate Limiting for Natural Killer Cell-Mediated Antibody-dependent Chronic Rejection
Abstract
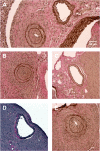
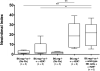
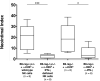
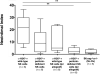
Natural killer (NK) cells are key components of the innate immune system. In murine cardiac transplant models, donor-specific antibodies (DSA), in concert with NK cells, are sufficient to inflict chronic allograft vasculopathy independently of T and B cells. In this study, we aimed to determine the effector mechanism(s) required by NK cells to trigger chronic allograft vasculopathy during antibody-mediated rejection. Specifically, we tested the relative contribution of the proinflammatory cytokine interferon gamma (IFN-γ) versus the contact-dependent cytotoxic mediators of perforin and the CD95/CD95L (Fas/Fas ligand [FasL]) pathway for triggering these lesions. C3H/HeJ cardiac allografts were transplanted into immune-deficient C57BL/6 rag-/- γc-/- recipients, who also received monoclonal anti-major histocompatibility complex (MHC) class I DSA. The combination of DSA and wild-type NK cell transfer triggered aggressive chronic allograft vasculopathy. However, transfer of IFN-γ-deficient NK cells or host IFN-γ neutralization led to amelioration of these lesions. Use of either perforin-deficient NK cells or CD95 (Fas)-deficient donors alone did not alter development of vasculopathy, but simultaneous disruption of NK cell-derived perforin and allograft Fas expression resulted in prevention of these abnormalities. Therefore, both NK cell IFN-γ production and contact-dependent cytotoxic activity are rate-limiting effector pathways that contribute to this form of antibody-induced chronic allograft vasculopathy.
Keywords: animal models: murine; basic (laboratory) research/science; cytokines/cytokine receptors; immunobiology; natural killer (NK) cells/NK receptors; rejection: antibody-mediated (ABMR); rejection: chronic.
© Copyright 2016 The American Society of Transplantation and the American Society of Transplant Surgeons.
Figures

Similar articles
-
The natural killer cell activating receptor, NKG2D, is critical to antibody-dependent chronic rejection in heart transplantation.Am J Transplant. 2021 Nov;21(11):3550-3560. doi: 10.1111/ajt.16690. Epub 2021 Jun 17. Am J Transplant. 2021. PMID: 34014614 Free PMC article.
-
Acute cardiac allograft rejection by directly cytotoxic CD4 T cells: parallel requirements for Fas and perforin.Transplantation. 2010 Jan 15;89(1):33-9. doi: 10.1097/TP.0b013e3181be6bc7. Transplantation. 2010. PMID: 20061916 Free PMC article.
-
Islet allograft rejection by contact-dependent CD8+ T cells: perforin and FasL play alternate but obligatory roles.Am J Transplant. 2007 Aug;7(8):1927-33. doi: 10.1111/j.1600-6143.2007.01889.x. Am J Transplant. 2007. PMID: 17617855
-
Cytolytic molecules in rejection.Curr Opin Organ Transplant. 2009 Feb;14(1):30-3. doi: 10.1097/MOT.0b013e32831c8462. Curr Opin Organ Transplant. 2009. PMID: 19337143 Review.
-
Structure and function of major histocompatibility complex class I antigens.Curr Opin Organ Transplant. 2010 Aug;15(4):499-504. doi: 10.1097/MOT.0b013e32833bfb33. Curr Opin Organ Transplant. 2010. PMID: 20613521 Free PMC article. Review.
Cited by
-
Bringing Clarity to the Murky Problem of Cardiac Allograft Vasculopathy.Am J Pathol. 2022 Jul;192(7):986-989. doi: 10.1016/j.ajpath.2022.05.002. Epub 2022 May 14. Am J Pathol. 2022. PMID: 35577009 Free PMC article. No abstract available.
-
Deletion of the activating NK cell receptor NKG2D accelerates rejection of cardiac allografts.Am J Transplant. 2017 Dec;17(12):3199-3209. doi: 10.1111/ajt.14467. Epub 2017 Sep 9. Am J Transplant. 2017. PMID: 28805342 Free PMC article.
-
Recent advances in allograft vasculopathy.Curr Opin Organ Transplant. 2017 Feb;22(1):1-7. doi: 10.1097/MOT.0000000000000370. Curr Opin Organ Transplant. 2017. PMID: 27898462 Free PMC article. Review.
-
The natural killer cell activating receptor, NKG2D, is critical to antibody-dependent chronic rejection in heart transplantation.Am J Transplant. 2021 Nov;21(11):3550-3560. doi: 10.1111/ajt.16690. Epub 2021 Jun 17. Am J Transplant. 2021. PMID: 34014614 Free PMC article.
-
Tocilizumab-Based Treatment of Microvascular Inflammation in Kidney Transplant Recipients: A Retrospective Study.Transpl Int. 2025 May 16;38:14502. doi: 10.3389/ti.2025.14502. eCollection 2025. Transpl Int. 2025. PMID: 40454296 Free PMC article.
References
-
- Kim WR, Lake JR, Smith JM, Skeans MA, Schladt DP, Edwards EB, et al. OPTN/SRTR 2013 Annual Data Report: liver. American journal of transplantation : official journal of the American Society of Transplantation and the American Society of Transplant Surgeons. 2015 Jan;15(Suppl 2):1–28. - PubMed
-
- Valapour M, Skeans MA, Heubner BM, Smith JM, Hertz MI, Edwards LB, et al. OPTN/SRTR 2013 Annual Data Report: lung. American journal of transplantation : official journal of the American Society of Transplantation and the American Society of Transplant Surgeons. 2015 Jan;15(Suppl 2):1–28. - PubMed
-
- Kandaswamy R, Skeans MA, Gustafson SK, Carrico RJ, Tyler KH, Israni AK, et al. OPTN/SRTR 2013 Annual Data Report: pancreas. American journal of transplantation : official journal of the American Society of Transplantation and the American Society of Transplant Surgeons. 2015 Jan;15(Suppl 2):1–20. - PubMed
-
- Matas AJ, Smith JM, Skeans MA, Thompson B, Gustafson SK, Stewart DE, et al. OPTN/SRTR 2013 Annual Data Report: kidney. American journal of transplantation : official journal of the American Society of Transplantation and the American Society of Transplant Surgeons. 2015 Jan;15(Suppl 2):1–34. - PubMed
-
- Colvin-Adams M, Smith JM, Heubner BM, Skeans MA, Edwards LB, Waller CD, et al. OPTN/SRTR 2013 Annual Data Report: heart. American journal of transplantation : official journal of the American Society of Transplantation and the American Society of Transplant Surgeons. 2015 Jan;15(Suppl 2):1–28. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

