Aberrant Calreticulin Expression in Articular Cartilage of Dio2 Deficient Mice
- PMID: 27163789
- PMCID: PMC4862667
- DOI: 10.1371/journal.pone.0154999
Aberrant Calreticulin Expression in Articular Cartilage of Dio2 Deficient Mice
Abstract
Objective: To identify intrinsic differences in cartilage gene expression profiles between wild-type- and Dio2-/--mice, as a mechanism to investigate factors that contribute to prolonged healthy tissue homeostasis.
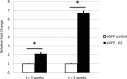
Methods: Previously generated microarray-data (Illumina MouseWG-6 v2) of knee cartilage of wild-type and Dio2 -/- -mice were re-analyzed to identify differential expressed genes independent of mechanical loading conditions by forced treadmill-running. RT-qPCR and western blot analyses of overexpression and knockdown of Calr in mouse chondro-progenitor cells (ATDC5) were applied to assess the direct effect of differential Calr expression on cartilage deposition.
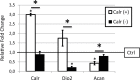
Results: Differential expression analyses of articular cartilage of Dio2-/- (N = 9) and wild-type-mice (N = 11) while applying a cutoff threshold (P < 0.05 (FDR) and FC > |1,5|) resulted in 1 probe located in Calreticulin (Calr) that was found significantly downregulated in Dio2-/- mice (FC = -1.731; P = 0.044). Furthermore, overexpression of Calr during early chondrogenesis in ATDC5 cells leads to decreased proteoglycan deposition and corresponding lower Aggrecan expression, whereas knocking down Calr expression does not lead to histological differences of matrix composition.
Conclusion: We here demonstrate that the beneficial homeostatic state of articular cartilage in Dio2-/- mice is accompanied with significant lower expression of Calr. Functional analyses further showed that upregulation of Calr expression could act as an initiator of cartilage destruction. The consistent association between Calr and Dio2 expression suggests that enhanced expression of these genes facilitate detrimental effects on cartilage integrity.
Conflict of interest statement
Figures


References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

