Protection From Glucocorticoid-Induced Osteoporosis by Anti-Catabolic Signaling in the Absence of Sost/Sclerostin
- PMID: 27163932
- PMCID: PMC8499032
- DOI: 10.1002/jbmr.2869
Protection From Glucocorticoid-Induced Osteoporosis by Anti-Catabolic Signaling in the Absence of Sost/Sclerostin
Abstract
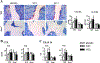
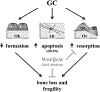
Excess of glucocorticoids, either due to disease or iatrogenic, increases bone resorption and decreases bone formation and is a leading cause of osteoporosis and bone fractures worldwide. Improved therapeutic strategies are sorely needed. We investigated whether activating Wnt/β-catenin signaling protects against the skeletal actions of glucocorticoids, using female mice lacking the Wnt/β-catenin antagonist and bone formation inhibitor Sost. Glucocorticoids decreased the mass, deteriorated the microarchitecture, and reduced the structural and material strength of bone in wild-type (WT), but not in Sost-/- mice. The high bone mass exhibited by Sost-/- mice is due to increased bone formation with unchanged resorption. However, unexpectedly, preservation of bone mass and strength in Sost-/- mice was due to prevention of glucocorticoid-induced bone resorption and not to restoration of bone formation. In WT mice, glucocorticoids increased the expression of Sost and the number of sclerostin-positive osteocytes, and altered the molecular signature of the Wnt/β-catenin pathway by decreasing the expression of genes associated with both anti-catabolism, including osteoprotegerin (OPG), and anabolism/survival, such as cyclin D1. In contrast in Sost-/- mice, glucocorticoids did not decrease OPG but still reduced cyclin D1. Thus, in the context of glucocorticoid excess, activation of Wnt/β-catenin signaling by Sost/sclerostin deficiency sustains bone integrity by opposing bone catabolism despite markedly reduced bone formation and increased apoptosis. This crosstalk between glucocorticoids and Wnt/β-catenin signaling could be exploited therapeutically to halt resorption and bone loss induced by glucocorticoids and to inhibit the exaggerated bone formation in diseases of unwanted hyperactivation of Wnt/β-catenin signaling. © 2016 American Society for Bone and Mineral Research.
Keywords: CORTICOSTEROIDS; GENETIC ANIMAL MODELS; MOLECULAR PATHWAYS - REMODELING; OSTEOPOROSIS; WNT/β-CATENIN/LRPS.
© 2016 American Society for Bone and Mineral Research.
Conflict of interest statement
Disclosures
All authors state that they have no conflicts of interest.
Figures





Comment in
-
Sclerostin Blockade-A Dual Mode of Action After All?J Bone Miner Res. 2016 Oct;31(10):1787-1790. doi: 10.1002/jbmr.2988. Epub 2016 Sep 14. J Bone Miner Res. 2016. PMID: 27597566 No abstract available.
Similar articles
-
SOST gene suppression stimulates osteocyte Wnt/β-catenin signaling to prevent bone resorption and attenuates particle-induced osteolysis.J Mol Med (Berl). 2023 May;101(5):607-620. doi: 10.1007/s00109-023-02319-2. Epub 2023 May 1. J Mol Med (Berl). 2023. PMID: 37121919 Free PMC article.
-
Disparate effects of sclerostin deletion on alveolar bone and cellular cementum in mice.J Periodontol. 2025 Jan;96(1):82-96. doi: 10.1002/JPER.24-0025. Epub 2024 Jul 16. J Periodontol. 2025. PMID: 39012429 Free PMC article.
-
Reversing LRP5-dependent osteoporosis and SOST deficiency-induced sclerosing bone disorders by altering WNT signaling activity.J Bone Miner Res. 2014 Jan;29(1):29-42. doi: 10.1002/jbmr.2059. J Bone Miner Res. 2014. PMID: 23901037
-
Involvement of WNT/β-catenin signaling in the treatment of osteoporosis.Calcif Tissue Int. 2013 Aug;93(2):121-32. doi: 10.1007/s00223-013-9749-z. Epub 2013 Jun 11. Calcif Tissue Int. 2013. PMID: 23748710 Review.
-
The role of Wnt signaling and sclerostin in the pathogenesis of glucocorticoid-induced osteoporosis.Curr Osteoporos Rep. 2014 Mar;12(1):90-7. doi: 10.1007/s11914-014-0197-0. Curr Osteoporos Rep. 2014. PMID: 24488619 Review.
Cited by
-
Glucocorticoid-induced osteoporosis: an update.Endocrine. 2018 Jul;61(1):7-16. doi: 10.1007/s12020-018-1588-2. Epub 2018 Apr 24. Endocrine. 2018. PMID: 29691807 Free PMC article. Review.
-
Decreasing miR-433-3p Activity in the Osteoblast Lineage Blunts Glucocorticoid-mediated Bone Loss.Endocrinology. 2025 Jan 6;166(2):bqaf008. doi: 10.1210/endocr/bqaf008. Endocrinology. 2025. PMID: 39820728
-
Bushen huoxue decoction inhibits RANKL-stimulated osteoclastogenesis and glucocorticoid-induced bone loss by modulating the NF-κB, ERK, and JNK signaling pathways.Front Pharmacol. 2022 Nov 18;13:1007839. doi: 10.3389/fphar.2022.1007839. eCollection 2022. Front Pharmacol. 2022. PMID: 36467086 Free PMC article.
-
Short-Term Glucocorticoid Treatment Reduces Circulating Sclerostin Concentrations in Healthy Young Men: A Randomized, Placebo-Controlled, Double-Blind Study.JBMR Plus. 2020 Jun 13;4(8):e10341. doi: 10.1002/jbm4.10341. eCollection 2020 Aug. JBMR Plus. 2020. PMID: 32803106 Free PMC article.
-
Plasma sclerostin levels are associated with nutritional status and insulin resistance but not hormonal disturbances in women with polycystic ovary syndrome.Arch Gynecol Obstet. 2020 Oct;302(4):1025-1031. doi: 10.1007/s00404-020-05656-6. Epub 2020 Jun 26. Arch Gynecol Obstet. 2020. PMID: 32592042 Free PMC article.
References
-
- Warriner AH, Saag KG. Glucocorticoid-related bone changes from endogenous or exogenous glucocorticoids. Curr Opin Endocrinol Diabetes Obes. 2013;20:510–6. - PubMed
-
- Necela BM, Cidlowski JA. Mechanisms of glucocorticoid receptor action in noninflammatory and inflammatory cells. Proc Am Thorac Soc. 2004;1:239–46. - PubMed
-
- Rhen T, Cidlowski JA. Antiinflammatory action of glucocorticoids—new mechanisms for old drugs. N Engl J Med. 2005;353:1711–23. - PubMed
-
- Tauchmanovà L, Pivonello R, Di Somma C, et al. Bone demineralization and vertebral fractures in endogenous cortisol excess: role of disease etiology and gonadal status. J Clin Endocrinol Metab. 2006;91:1779–84. - PubMed
-
- Vestergaard P, Lindholm J, Jorgensen JO, et al. Increased risk of osteoporotic fractures in patients with Cushing’s syndrome. Eur J Endocrinol. 2002;146:51–6. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials

