Expression Profile of Human Fc Receptors in Mucosal Tissue: Implications for Antibody-Dependent Cellular Effector Functions Targeting HIV-1 Transmission
- PMID: 27164006
- PMCID: PMC4862624
- DOI: 10.1371/journal.pone.0154656
Expression Profile of Human Fc Receptors in Mucosal Tissue: Implications for Antibody-Dependent Cellular Effector Functions Targeting HIV-1 Transmission
Abstract
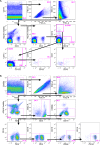
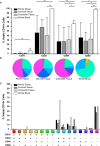
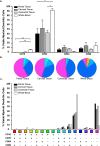
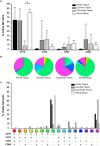
The majority of new Human Immunodeficiency Virus (HIV)-1 infections are acquired via sexual transmission at mucosal surfaces. Partial efficacy (31.2%) of the Thai RV144 HIV-1 vaccine trial has been correlated with Antibody-dependent Cellular Cytotoxicity (ADCC) mediated by non-neutralizing antibodies targeting the V1V2 region of the HIV-1 envelope. This has led to speculation that ADCC and other antibody-dependent cellular effector functions might provide an important defense against mucosal acquisition of HIV-1 infection. However, the ability of antibody-dependent cellular effector mechanisms to impact on early mucosal transmission events will depend on a variety of parameters including effector cell type, frequency, the class of Fc-Receptor (FcR) expressed, the number of FcR per cell and the glycoslyation pattern of the induced antibodies. In this study, we characterize and compare the frequency and phenotype of IgG (CD16 [FcγRIII], CD32 [FcγRII] and CD64 [FcγRI]) and IgA (CD89 [FcαR]) receptor expression on effector cells within male and female genital mucosal tissue, colorectal tissue and red blood cell-lysed whole blood. The frequency of FcR expression on CD14+ monocytic cells, myeloid dendritic cells and natural killer cells were similar across the three mucosal tissue compartments, but significantly lower when compared to the FcR expression profile of effector cells isolated from whole blood, with many cells negative for all FcRs. Of the three tissues tested, penile tissue had the highest percentage of FcR positive effector cells. Immunofluorescent staining was used to determine the location of CD14+, CD11c+ and CD56+ cells within the three mucosal tissues. We show that the majority of effector cells across the different mucosal locations reside within the subepithelial lamina propria. The potential implication of the observed FcR expression patterns on the effectiveness of FcR-dependent cellular effector functions to impact on the initial events in mucosal transmission and dissemination warrants further mechanistic studies.
Conflict of interest statement
Figures



Similar articles
-
Broadly Neutralizing Antibodies Display Potential for Prevention of HIV-1 Infection of Mucosal Tissue Superior to That of Nonneutralizing Antibodies.J Virol. 2016 Dec 16;91(1):e01762-16. doi: 10.1128/JVI.01762-16. Print 2017 Jan 1. J Virol. 2016. PMID: 27795431 Free PMC article.
-
IgA Targeting Human Immunodeficiency Virus-1 Envelope gp41 Triggers Antibody-Dependent Cellular Cytotoxicity Cross-Clade and Cooperates with gp41-Specific IgG to Increase Cell Lysis.Front Immunol. 2018 Mar 29;9:244. doi: 10.3389/fimmu.2018.00244. eCollection 2018. Front Immunol. 2018. PMID: 29651286 Free PMC article.
-
Recombinant gp120 vaccine-induced antibodies inhibit clinical strains of HIV-1 in the presence of Fc receptor-bearing effector cells and correlate inversely with HIV infection rate.J Immunol. 2007 May 15;178(10):6596-603. doi: 10.4049/jimmunol.178.10.6596. J Immunol. 2007. PMID: 17475891 Clinical Trial.
-
The role of HIV-specific antibody-dependent cellular cytotoxicity in HIV prevention and the influence of the HIV-1 Vpu protein.AIDS. 2015 Jan 14;29(2):137-44. doi: 10.1097/QAD.0000000000000523. AIDS. 2015. PMID: 25396265 Review.
-
Antibody Functional Assays as Measures of Fc Receptor-Mediated Immunity to HIV - New Technologies and their Impact on the HIV Vaccine Field.Curr HIV Res. 2017;15(3):202-215. doi: 10.2174/1570162X15666170320112247. Curr HIV Res. 2017. PMID: 28322167 Free PMC article. Review.
Cited by
-
Physiological and Pathological Inflammation Induced by Antibodies and Pentraxins.Cells. 2021 May 12;10(5):1175. doi: 10.3390/cells10051175. Cells. 2021. PMID: 34065953 Free PMC article. Review.
-
Prospects from systems serology research.Immunology. 2018 Mar;153(3):279-289. doi: 10.1111/imm.12861. Epub 2017 Dec 1. Immunology. 2018. PMID: 29139548 Free PMC article. Review.
-
Broadly Neutralizing Antibodies Display Potential for Prevention of HIV-1 Infection of Mucosal Tissue Superior to That of Nonneutralizing Antibodies.J Virol. 2016 Dec 16;91(1):e01762-16. doi: 10.1128/JVI.01762-16. Print 2017 Jan 1. J Virol. 2016. PMID: 27795431 Free PMC article.
-
Antibody Fc-chimerism and effector functions: When IgG takes advantage of IgA.Front Immunol. 2023 Feb 2;14:1037033. doi: 10.3389/fimmu.2023.1037033. eCollection 2023. Front Immunol. 2023. PMID: 36817447 Free PMC article. Review.
-
Transmitted/founder (T/F) HIV-1 derived from sexual contact exhibits greater transmission fitness in human cervical tissue than T/F HIV-1 from blood-to-blood contact: Unique glycan profiles on T/F envelopes associated with transmission phenotypes.PLoS Pathog. 2025 May 23;21(5):e1013177. doi: 10.1371/journal.ppat.1013177. eCollection 2025 May. PLoS Pathog. 2025. PMID: 40408432 Free PMC article.
References
-
- Haynes BF, Gilbert PB, McElrath MJ, Zolla-Pazner S, Tomaras GD, Alam SM, et al. Immune-correlates analysis of an HIV-1 vaccine efficacy trial. The New England journal of medicine. 2012;366(14):1275–86. Epub 2012/04/06. 10.1056/NEJMoa1113425 ; PubMed Central PMCID: PMCPmc3371689. - DOI - PMC - PubMed
-
- Tomaras GD, Ferrari G, Shen X, Alam SM, Liao HX, Pollara J, et al. Vaccine-induced plasma IgA specific for the C1 region of the HIV-1 envelope blocks binding and effector function of IgG. Proceedings of the National Academy of Sciences of the United States of America. 2013;110(22):9019–24. Epub 2013/05/11. 10.1073/pnas.1301456110 ; PubMed Central PMCID: PMCPmc3670311. - DOI - PMC - PubMed
-
- Yates NL, Liao HX, Fong Y, deCamp A, Vandergrift NA, Williams WT, et al. Vaccine-induced Env V1-V2 IgG3 correlates with lower HIV-1 infection risk and declines soon after vaccination. Science translational medicine. 2014;6(228):228ra39 Epub 2014/03/22. 10.1126/scitranslmed.3007730 ; PubMed Central PMCID: PMCPmc4116665. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

